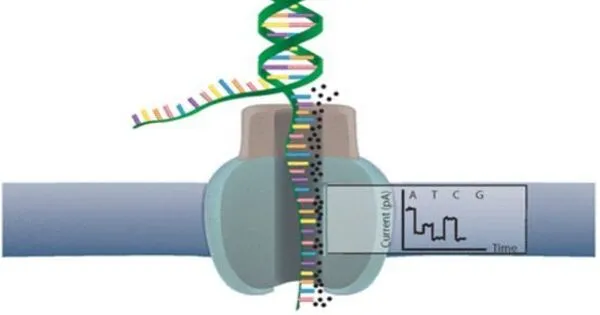
Nanopore sequencing and DNA barcoding are two promising genomics technologies that have the potential to profoundly improve personalized medicine. Nanopore sequencing is a cutting-edge DNA sequencing method that allows for real-time, single-molecule sequencing of DNA, RNA, and other biomolecules. It operates by passing a DNA strand through a nanopore and using variations in electrical current to decode the sequence of nucleotides.
With the ability to map hundreds of indicators at once, a new technology could revolutionize diagnostics for illnesses such as heart disease and cancer. Many diseases are currently diagnosed using blood tests that look for one biomarker (such as a protein or other tiny molecule) or, at most, a number of biomarkers of the same type.
The novel technology, developed by Imperial College London scientists in partnership with Oxford Nanopore Technologies (Oxford Nanopore), can analyze dozens of different types of biomarkers at the same time. This could potentially allow medics to learn more about a patient’s illness.
Current testing for heart failure, for example, seeks for a couple of common proteins to determine whether the problem exists. Additionally, the novel approach detected 40 different types of miRNA molecules, which have the potential to be exploited as a new class of biomarkers. It can test proteins, tiny molecules such as neurotransmitters, and miRNA from the same clinical sample at the same time, providing comprehensive data for a more exact diagnosis.
There are many different ways to arrive at heart failure. Our test will hopefully provide a low-cost and rapid way to find this out and help guide treatment options. With less than a milliliter of blood, this type of outcome is achievable.
Caroline Koch
The findings of proof-of-concept research employing the novel test on the blood of healthy individuals were reported today in Nature Nanotechnology.
“There are many different ways to arrive at heart failure,” said co-first author Caroline Koch of Imperial’s Department of Chemistry. “Our test will hopefully provide a low-cost and rapid way to find this out and help guide treatment options. With less than a milliliter of blood, this type of outcome is achievable. It’s also a very customizable technology, which might be used to detect the characteristics of diseases such as cancer and neurodegenerative ailments by modifying the target biomarkers.”
Co-first author Ben Reilly-O’Donnell, from the National Heart and Lung Institute at Imperial, added: “The ability to monitor different types of molecules at the same time, in the same sample, offers a distinct advantage over traditional analysis methods.”
DNA barcoding
The blood sample is mixed with DNA ‘barcodes’ for the test to work. These are short DNA sequences that encode a distinct probe designed to connect to a separate biomarker. After combining the sample and barcodes, the resulting solution is pumped into the MinION, a low-cost handheld device previously created by Oxford Nanopore.
The Oxford Nanopore gadget contains a flow cell with an array of nanopores (extremely small holes) that can read the electrical signature from each DNA barcode that passes through them. A machine-learning algorithm interprets the complicated electrical signal produced by the gadget to determine the type and concentration of each biomarker present in the sample.
The DNA barcodes used for each test can be made to order, specifically for the biomarkers that need to be analysed to characterize the disease being tested for. Using DNA barcodes also removes the need for complex and time-consuming sample preparation, which can also introduce sample bias.
“Working with Oxford Nanopore Technologies, we were able to take their existing platform and innovate how it can be used, with the addition of DNA barcodes and machine learning to understand the results,” said lead researcher Professor Joshua Edel from Imperial’s Department of Chemistry.
Dr. Alex Ivanov, co-lead researcher from Imperial’s Department of Chemistry, stated, “In principle, we are close to enabling a technology suitable for clinics, where, in the long run, we hope it could provide a wealth of individualized information for patients with a variety of conditions.”
Future directions
The team is now working with clinical samples from heart failure patients to validate the results after demonstrating that this technology can successfully measure 40 miRNA molecules in healthy patient blood. This type of testing could also assist clinicians in establishing individual patient baselines for common blood indicators.
The technology could assist speed up diagnosis in two ways: it can measure multiple biomarkers at once and it can also help uncover novel biomarkers. While just a few biomarkers have been verified for identifying cardiac disease, the team was able to see which of these are significant and could be validated with additional testing by evaluating 40 miRNA types of interest at the same time.