Changes known as epigenetic adjustments play a significant part in disease advancement. Having the option to examine them rapidly and dependably could contribute significantly to the further advancement of customized treatment. An examination group from the Institute of Physiology at the University of Freiburg has now prevailed with regards to portraying the compound changes in proteins that are common for epigenetic changes utilizing nanopore examination. The analysts have distributed their exploration in the Journal of the American Chemical Society (JACS).
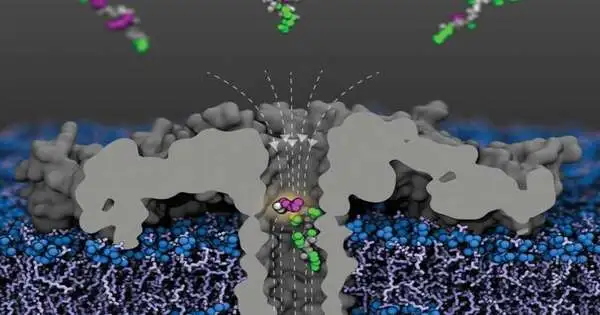
Lately, nanopores have turned into a widely used device for the examination of particles. Because of their unique properties, they permit the design of particles to be examined inside parts of a second: As rotundly organized proteins, nanopores structure small channels a couple of millionths of a millimeter (nanometer) in width that can be implanted in biomembranes.
“For the tests, we apply a steady voltage across the film so particles from the encompassing medium move through the pore. “This makes a steady, exactly quantifiable electric flow,” makes sense to Prof. Dr. Jan C. Behrends from the Faculty of Medicine at the University of Freiburg, in whose lab the now-distributed tests occurred. Nonetheless, when a particle moves into the pore, the current is impeded; the bigger the atom, the more firmly it is hindered as well.
A protein in the examination spotlight: H4
With regards to the tests currently distributed, the Freiburg researchers gave themselves over to the examination of the alleged histone protein H4. This protein is solidly connected with DNA in all cells with a core and is one of the most explored focuses of epigenetic changes. The A locale at the N-terminal end of the protein is especially impacted by these changes.
“Acetylation at K16, for example, is vital for human development, while methylation at K12 has a role in the formation of various prostate and lung malignancies, according to the newest studies from Medical Center—University of Freiburg.”
Prof. Dr. Jan C. Behrends from the Faculty of Medicine at the University of Freiburg
“The protein grouping there contains the amino corrosive lysine a few times,” Behrends makes sense of. Acetyl or methyl groups, for instance, can be joined to these lysines, which are assigned K8, K12, and K16 as per their position in the protein chain, as a feature of epigenetic changes. Which compound change happens at which lysine position is certainly of clinical significance, as the Freiburg physiologist brings up. “Acetylation at K16, for instance, is significant for human turn of events, while methylation at K12 plays a part in the improvement of some prostate and lung cancers, as per the most recent outcomes from the Medical Center—University of Freiburg.”
Recognizing changes with the assistance of a nanopore
In their tests, Behrends and his group were currently able to plainly recognize H4 parts regardless of acetylation, as well as pieces with one or a few acetylations. Furthermore, they were successful in demonstrating that the nanopore they used was sensitive to the site of acetylation: histone parts with an acetyl bunch at K8 obstructed current through the pore more firmly than those acetylated at K12, and thus more emphatically than those with a K16 acetylation.
“This sort of awareness is amazing in that these parts are indistinguishable regarding their mass and all out volume,” Behrends says. Hence, the pore current seems, by all accounts, to be delicate not exclusively to the size but in addition to the state of the atom. It was similarly simple to recognize the various variations of doubly acetylated histone parts—K8 and K12, K8 and K16, and K12 and K16—once more, in spite of the indistinguishable mass. H4 parts methylated to various degrees and at various positions likewise impeded the ongoing through the pore to various degrees, albeit not as plainly as the acetylated variations.
“We have had the option to show interestingly through our tests that nanopore examination permits us to recognize atoms by their size, but in addition by their shape,” says concentrate on pioneer Behrends. Subatomic element simulations led by the research group led by Aleksei Aksimentiev from the University of Illinois in the United States — who is also involved in the study — show that a profoundly inhomogeneous electric field inside the pore plays a significant role in this effect.
Future vision: Optimized clinical diagnostics
While the sequencing of DNA utilizing nanopores is now settled and marketed, the advancement of nanopore-based examination of proteins is simply starting, Behrends says. “The trouble with sequencing proteins is that these are atoms with non-uniform charge designs.” While DNA, which is adversely charged, moves directionally in the electric field and can hence be gotten through the pore base by base, proteins comprise of building blocks made of the amino acids with various charges. Thus, coordinated development in the electric field and “checking” amino corrosive by amino corrosive is absurd. The Freiburg researchers hence depended on an alternate methodology for their tests. Rather than a pore with a short choke, as utilized in DNA sequencing, they utilized a tailor-made pore with a sort of sub-atomic snare. “This permitted the whole protein part to be caught immediately,” says Behrends.
It isn’t yet clear up to which part size this sort of examination can be utilized. Nonetheless, extra tests show that the strategy will also be reasonable for the examination of the H4 parts recently utilized in epigenetic research. These contain 14 amino acids rather than the ten used here, and are currently being tested for epigenetic changes using pair mass spectrometry, a highly complex method.The analysts trust that the nanopores will make the examination a lot easier, quicker, and more savvy, and that it tends to be done near the patient.
The advancement of nanopore examination of proteins for clinical diagnostics and its application in significant items and administrations is also one of the focal tasks of the recently approved BMBF Cluster4Future nanodiagBW, which Behrends co-directs with Prof. Dr. Felix von Stetten of the Hahn-Schickard-Gesellschaft, which is the task’s lead.
More information: Tobias Ensslen et al, Resolving Isomeric Posttranslational Modifications Using a Biological Nanopore as a Sensor of Molecular Shape, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c06211
Journal information: Journal of the American Chemical Society