As a result of landfilling, animal cultivation, coal mining, and other human activities, methane outflows are one of the vital drivers of environmental change.
For quite a long time, researchers have attempted to foster cheap ways of using methane—which is the essential part of petroleum gas—without likewise creating carbon dioxide, the most bountiful ozone-harming substance in the Earth’s air.
Among the potential arrangements is dry changing, a cycle that can possibly change both methane and carbon dioxide into compound feedstocks, which are natural substances that can be utilized to make or handle different items.
However, for dry changing to be financially viable, greater than ever impetus is required.
“To accomplish the Paris Agreement’s aim of carbon neutrality, we must execute major reforms in both energy generation and chemical feedstock manufacturing,”
Mark Swihart, Ph.D., SUNY Distinguished Professor
In two University at Buffalo-led studies published in June — one in Chem Catalysis and the other in Angewandte Chemie — scientists report another creation strategy for making nickel-based impetuses that could overcome long-standing challenges.
To meet the goals of the Paris Agreement and to achieve carbon impartiality, we should implement many changes to both energy and the creation of compound feedstocks,” says the examinations’ lead creator, Mark Swihart, Ph.D., SUNY Distinguished Professor and chair of the Department of Chemical and Biological Engineering in the UB School of Engineering and Applied Sciences.
Shuo Liu, a Ph.D. applicant in Swihart’s lab, is the first creator of the examinations.
Co-creators with UB ties incorporate Satyarit Rao, Mihir Shah, Jilun Wei, Kaiwen Chen, and Zhengxi Xuan; as well as Eleni A. Kyriakidou, Ph.D., partner teacher of compound and organic design at UB; and Junjie Chen, Ph.D., a postdoctoral researcher at Stanford University who got a Ph.D. in Kyriakidou’s lab.
Other co-creators include Jeffery J. Metropolitan, Ph.D., head of the Inorganic Nanostructures Facility at the Molecular Foundry of the Lawrence Berkeley National Lab, and Chaochao Dun, Ph.D., a postdoctoral researcher at Urban’s lab.
Swihart makes sense of that dry changing of methane isn’t monetarily feasible utilizing existing nickel-based impetuses, which quit working on the grounds that their chemically dynamic particles become covered with carbon stores (coking) or join into bigger, less dynamic particles (sintering). The most promising impetuses, likewise, require complex creation methods.
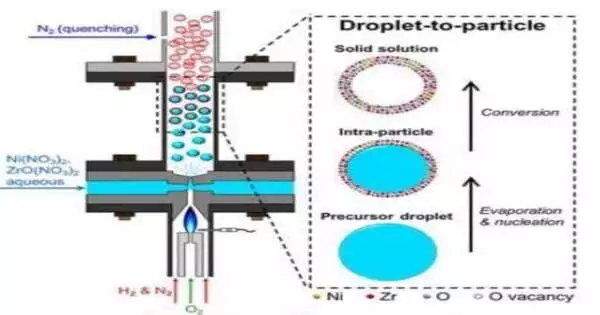
To address this issue, the research team devised a one-step spray cycle to generate low-cost and elite execution impetus.The cycle depends on a novel fire reactor created in Swihart’s lab.
The group utilized the reactor to make small round particles called nanoshells that oppose both coking and sintering.
In the Chem Catalysis study, that’s what the group detailed. Throughout 500 hours, the impetuses stayed viable, changing over 98% of methane into engineered gas, or syngas, which is a combination of hydrogen and carbon monoxide that can hence be utilized to create various compound items.
In a subsequent report, the group utilized the reactor to create a new mesoporous silica material that has a surface region that surpasses 1,000 square meters for each gram. The group likewise made a strategy to store nickel or other nanoparticles inside the mesoporous silica—a process known as in-situ statement.
As revealed in Angewandte Chemie, the mesoporous silica impetus changed over 97% of methane for north of 200 hours.
According to this headway, Swihart gives a pathway not exclusively to further developed impetuses for dry changing of methane, yet for some other earthly and monetarily useful responses.
More information: Shuo Liu et al, A General Route to Flame Aerosol Synthesis and In Situ Functionalization of Mesoporous Silica, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202206870
Shuo Liu et al, Producing ultrastable Ni-ZrO2 nanoshell catalysts for dry reforming of methane by flame synthesis and Ni exsolution, Chem Catalysis (2022). DOI: 10.1016/j.checat.2022.05.013