Researchers in New Zealand and Australia working at the degree of iotas have made something startling: small metallic snowflakes.
Why’s that so huge? Since cajoling individual iotas to coordinate is prompting an upset in designing and innovation through nanomaterials, (Also, it is cool to make snowflakes.)
Nanoscale designs can help electronic assembly, make materials more grounded at this point and therefore lighter, or help natural clean-ups by restricting poisons.
To make metallic nanocrystals, New Zealand and Australian researchers have been trying different things with gallium, a delicate, shiny metal that is utilized in semiconductors and, bizarrely, liquifies at just above room temperature.
Their outcomes were simply detailed in the diary.
“What we are discovering is that the structure of liquid gallium is quite critical. That’s unusual because we normally conceive of liquids as being either unstructured or only randomly organized.”
Professor Nicola Gaston
Teachers Nicola Gaston and Dr. Steph Lambie of Waipapa Taumata Rau, College of Auckland, and Dr. Krista Steenbergen of Te Herenga Waka, Victoria College of Wellington collaborated with Australian partners led by Teacher Kourosh Kalantar-Zadeh of the College of New South Ribs.
The Australian group worked in the lab with nickel, copper, zinc, tin, platinum, bismuth, silver, and aluminum. Metals were broken up by gallium at high temperatures. When cooled, the metallic gems arose while the gallium stayed fluid.
The New Zealand group, which is part of the MacDiarmid Foundation for Cutting Edge Materials and Nanotechnology, a public Focus of Exploration Greatness, recreated subatomic elements to figure out why variously shaped gems emerge from various metals.
“We are discovering that the design of the fluid gallium is vital,” says Gaston. “That is novel because we normally think of fluids as lacking design or being arbitrarily organized.”
Connections between the atomistic designs of the various metals and the fluid gallium make diversely formed gems arise, the researchers showed.
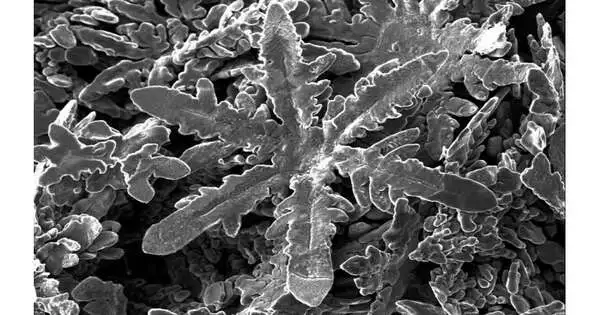
The gems included blocks, bars, hexagonal plates, and zinc snowflake shapes. The six-fanned balance of zinc, with every iota encompassed by six neighbors at identical distances, represents the snowflake plan.
“Rather than hierarchical ways to deal with shaping nanostructures—bby removing material—tthis granular perspective depends on iotas’ self-gathering,” says Gaston. “This is the way nature makes nanoparticles and is both less inefficient and considerably more exact than hierarchical techniques.”
She says the exploration has opened up a new, neglected pathway for metallic nanostructures. “There’s likewise something cool about making a metallic snowflake.”
More information: Shuhada A. Idrus-Saidi et al, Liquid metal synthesis solvents for metallic crystals, Science (2022). DOI: 10.1126/science.abm2731
Journal information: Science