Huntington’s illness, a lethal, acquired neurodegenerative condition, is brought about by a hereditary mistake present upon entering the world. Its side effects frequently don’t start until late adulthood. Researchers at Washington College Institute of Medication in St. Louis have been attempting to comprehend how the maturing system sets off the beginning of side effects, with the assumption that such information could highlight medicines that defer or forestall neurodegeneration.
With that in mind, another review from Washington College shows that as patients age, the illness slowly impedes a significant cell housekeeping process called autophagy, which is responsible for killing waste from cells. This housekeeping is huge in Huntington’s on the grounds that the development of waste in a particular sort of neuron prompts such cells’ troublesome passings.
The analysts likewise showed that improving the autophagy pathway in neurons that were made from skin cells of Huntington’s patients shields those cells from passing on.
“Our analysis illustrates how aging causes a loss of the critical mechanism of autophagy—and hints at how we can try to restore this vital function in order to delay or perhaps prevent Huntington’s disease,”
Senior author Andrew S. Yoo, Ph.D.
“Our review uncovers how maturing triggers a deficiency of the vital course of autophagy—and indicates how we could attempt to reestablish this significant capability, determined to defer or, in any event, forestall Huntington’s illness,” said senior creator Andrew S. Yoo, Ph.D., a Washington College teacher of formative science.
The review, which was published on October 27 in the journal Nature Neuroscience, may also provide clues to understanding mental degeneration in general.
Huntington’s illness obliterates a particular sort of synapse called medium-sharp neurons, the deficiency of which causes compulsory muscle development, impeded emotional wellness, and mental degradation. Patients commonly live around 20 years after indications of the illness initially show up.
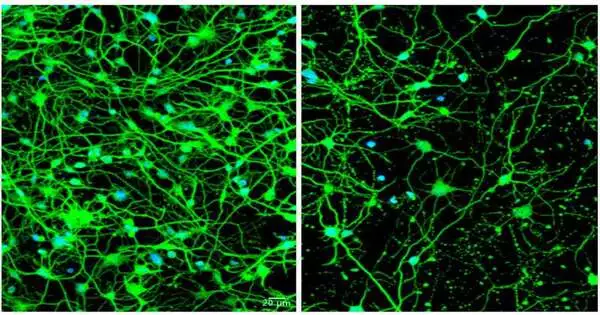
For this review, the scientists reinvented patients’ skin cells into medium-sharp neurons utilizing a method they fostered that permits grown-up skin cells to be changed straightforwardly into different kinds of synapses, contingent upon the particular recipe of flagging particles to which the skin cells are uncovered. More normal methods include utilization of immature microorganisms—yet undifferentiated organisms reset the cells’ natural clocks to an early formative state, which isn’t helpful while concentrating on illnesses that become suggestive in adulthood.
“We gathered skin cell tests from various patients at a range of ages and displayed the illness when side effects were created, which permitted us to recognize the distinctions between more youthful and more seasoned patients with Huntington’s sickness,” Yoo said. “We realized there should be some change that happens as patients age. They all have a hereditary change in the Huntingtin quality. We needed to find the contrast between youthful patients who have no side effects and more seasoned patients who effectively give indications of the illness.
Yoo and his partners, including co-first creators Youngmi Goodness, Ph.D., and Seongwon Lee, Ph.D., both staff researchers in Yoo’s lab, found that medium-sharp neurons reinvented from skin cells of more seasoned patients with suggestive Huntington’s delivered elevated degrees of a microRNA particle called miR-29b-3p.
These undeniable levels were not seen in younger Huntington’s patients or in reinvented neurons from healthy people of all ages.The examiners showed that the microRNA set off a chain of events that included impeding autophagy in these cells. At the point when the skin cells finished the change into neurons, they started creating the risky microRNA, autophagy dialed back, and the cells started passing on.
The analysts proceeded to show that diminishing levels of this microRNA permitted autophagy to proceed and shielded the neurons from passing on. Likewise, they found that improving autophagy with a substance compound called G2 shielded the sick neurons from death. As the analysts expanded the portion of G2, the security from cell demise improved too.
G2 is derived from a series of analogs discovered in the labs of co-creators David Perlmutter, MD, chief bad habit chancellor for clinical endeavors, the George and Song Bauer Dignitary of the Institute of Medication, and the Spencer T. Ann W. Olin Recognized Teacher; Gary Silverman, MD, Ph.D., the Harriet B. Spoehrer Teacher and head of the Branch of Pediatrics; and Stephen C. Pak, Ph.D
G2 was discovered through high throughput evaluating for autophagy enhancer sedates that could address the cell accumulation of variation alpha-1-antitrypsin Z, which causes liver disease in alpha-1-antitrypsin deficiency (ATD).The G2 mixtures could hence address alluring contenders for forestalling neurodegeneration in Huntington’s illness, liver sickness in alpha-1-antitrypsin lack and maybe different illnesses in which variant amassing of misfolded proteins is harmful to cells.
The focus also revealed what could be a tempting hint for comprehending mental degeneration in typical maturing.While contrasting the suggestive neurons with pre-suggestive neurons and with solid neurons from both youthful and more seasoned grown-ups, the analysts found that the neurons of sound more seasoned grown-ups created somewhat raised levels of the unsafe microRNA, yet in far more modest sums than the neurons of indicative Huntington’s illness patients.
The review proposes that even in typical, sound-maturing, medium-sharp neurons slowly produce low levels of this microRNA, which might impede autophagy’s solid cell housekeeping.
“By displaying various stages of the illness across the life expectancy, we can see how maturing plays a role in sickness onset,” Yoo explained.
“With that data, we can start to search for ways of deferring that beginning. Our study likewise proposes that the setting off atom for the beginning of Huntington’s illness might play some part in the age-related decrease in neuronal capability by and large. “Understanding the part of maturing that sets off neurodegeneration might assist in growing new systems for treatment and avoidance of Huntington’s illness and other neurodegenerative circumstances that develop at more seasoned ages.”
Yoo and his group are also working with different partners utilizing their cell reinventing method to examine types of Alzheimer’s illness, tauopathy, and other neurodegenerative circumstances.
More information: Young Mi Oh et al, Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy, Nature Neuroscience (2022). DOI: 10.1038/s41593-022-01185-4
Journal information: Nature Neuroscience