Osteoporosis and other diseases make it even harder for people to regenerate their bones as they get older. Researchers are looking for new treatments that make it easier for bones to grow back in order to help the aging population.
Now, a group of researchers from the Max Bergmann Center for Biomaterials (MBC) and the Biotechnology Center (BIOTEC) at the Medical Faculty of the TU Dresden have created novel bio-inspired molecules that help mice regenerate their bones. The findings were presented and discussed in the journal Biomaterials.
The capacity for bone regrowth declines with age. Fractures take longer to heal, which is made worse by conditions like osteoporosis. The aging population faces a serious health issue as a result of this, and society faces an increasing socioeconomic burden as a result. Researchers are looking for novel therapeutic strategies that can boost bone regeneration in order to assist in the fight against this problem.
“We used molecular modeling to create new structures that mimic important receptor interactions with both proteins. We intended this bond to be stronger than their normal interactions. In this sense, our new compounds would simultaneously hijack the proteins and efficiently switch them off in order to activate bone regeneration.”
Prof. Maria Teresa Pisabarro at the Biotechnology Center (BIOTEC) of TU Dresden.
A group of researchers from Dresden utilized PC demonstrations and recreations to plan novel bio-propelled particles to improve bone recovery in mice. Biomaterials can incorporate the new molecules, and bone defects can be treated locally with them. Glycosaminoglycans, which are long-chained sugars like heparin or hyaluronic acid, serve as the basis for these new molecules.
A sweet solution for an old bone “We now know a distinct molecular pathway that regulates bone formation and repair thanks to the work of our group and other researchers. According to Prof. Lorenz Hofbauer, “The big challenge for developing drugs that improve bone healing is to efficiently turn off both of these proteins, which act as brake signals, at the same time.” In fact, “we can narrow it down to two proteins that work together to block bone regeneration, sclerostin and Dickkopf-1.”
This challenge required an interdisciplinary approach. The Underlying Bioinformatics bunch, driven by Prof. Maria Teresa Pisabarro at the Biotechnology Place (BIOTEC) of TU Dresden, and the Utilitarian Biomaterials bunch, driven by PD Dr. Vera Hintze at the Maximum Bergmann Focal Point of Biomaterials (MBC), Establishment of Materials Study of TU Dresden, consolidated their ability with bone master Prof. Lorenz Hofbauer at the Clinical Personnel of TU Dresden.
“We have used computer simulations to study how proteins that control bone formation interact with their receptors for several years. All of this is done to create new molecules that effectively disrupt these interactions. We worked in pairs between the PC and the seat, planning new atoms and testing them, taking care of the outcomes back to our sub-atomic models, and getting more familiar with the atomic properties expected for our objective,” makes sense of Prof. Pisabarro.
At last, the group in Lorenz Hofbauer’s Bone Lab utilized a biomaterial stacked with the new particles on bone deformities in mice to test their viability. The gathering found that materials containing the clever particles beat the standard biomaterial and upgraded bone recovery by up to half, which shows their true capacity for working on bone recovery.
Chain of value-adding: From the computer to the laboratory bench and back again, a multidisciplinary team employed rational drug design to produce novel molecules with tailored properties and few side effects. The team was able to identify a number of potential candidates with the greatest potential for turning off the proteins that prevent bone regeneration by utilizing computational methods to predict and refine the properties of the designed molecules.
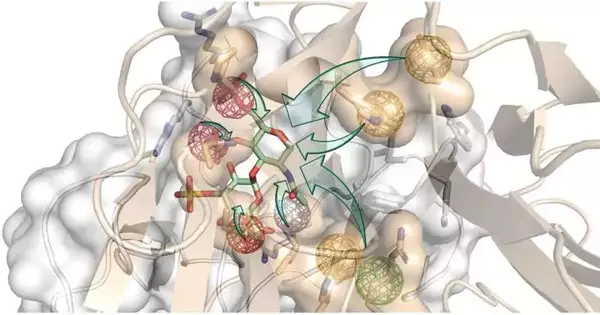
The expertise of the Pisabarro group made it possible to thoroughly examine the three-dimensional (3D) structures of the two proteins that prevent bone regeneration. Because of this, they were able to create a three-dimensional model of their interaction with their receptors and locate what are known as “hot spots,” which are particular physicochemical and dynamic properties that are necessary for the biological interaction to take place.
“We designed new structures that modeled relevant receptor interactions with both proteins using molecular modeling.” Our goal was for this binding to be more powerful than their normal interactions. Prof. Pisabarro explains, “In this manner, our novel molecules would simultaneously hijack the proteins and effectively turn them off to turn on the bone regeneration.”
According to PD Dr. Hintze, “our colleagues at the Free University of Berlin synthesized the molecules designed by Pisabarro’s group and then analyzed their protein binding properties via biophysical interaction analysis.” We were able to measure the molecules’ interference with the proteins’ natural receptor binding as well as their binding strength with the proteins. As a result, we might be able to demonstrate empirically how well each of the small molecules turned off the inhibitory proteins.” Hofbauer’s bunch then, at that point, tried the organic pertinence of these collaborations in a cell culture model and later in mice.
The results of such iterative testing are a valuable asset that can be used to improve the Pisabarro group’s existing molecular models and direct the creation of new molecules in the future. Additionally, this strategy ensures that animal research is minimized and only included in the project’s final phase.
Preclinical development has taken an exciting leap forward with the team’s findings. The recently planned atoms might actually be utilized to switch off the proteins that block bone recovery and lead to the advancement of novel, more successful medicines for bone cracks and other bone-related conditions.
More information: Gloria Ruiz-Gómez et al, Rational engineering of glycosaminoglycan-based Dickkopf-1 scavengers to improve bone regeneration, Biomaterials (2023). DOI: 10.1016/j.biomaterials.2023.122105