In general, Li-particle batteries (LIBs) are generally utilized batteries that help present day ITC society, including cell phones and EVs. LIBs are repeatedly charged and released by Li-particles passing between the positive and negative cathodes, with the Li-particle electrolyte acting as a conduit for the particles.
Regularly, natural electrolytes, for example, fluid ethylene carbonate (EC) and its gels, have been utilized as the Li-particle electrolyte because of their voltage obstruction and ionic conductivity. In any case, as fluids and gels are combustible, a change to more secure polymeric strong electrolytes is best.
Polymeric strong electrolytes like polyethylene glycol (Stake) have been proposed as effective Li-particle electrolytes. Nonetheless, stake-based polymer electrolytes solidify close to room temperature, bringing about a critical drop in Li-particle conductivity to around 10-6 S/cm at room temperature.
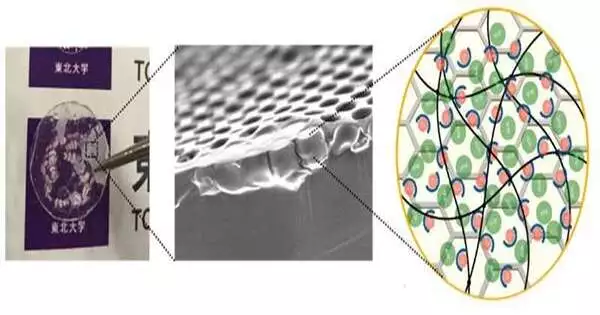
To tackle this issue, an examination group has designed another kind of polymeric strong electrolyte by joining a permeable polymer layer with a few micron pores and a photocross-linkable polyethylene glycol Stake based polymer electrolyte. The polymeric strong electrolyte has a wide possible window (4.7 V), a high Li-particle conductivity in the OK S/cm class, which is comparable to a fluid and adequate for down-to-earth use, and a high Li-particle transaction number (0.39).
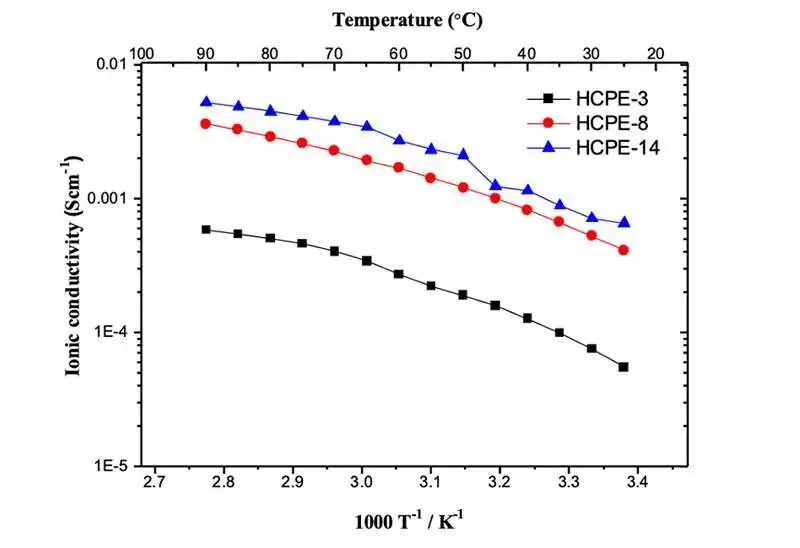
The effect of temperature on ionic conductivities of various pore size honeycomb film composite electrolytes.Photographers: Grewal and YabuLi-Particles moving in the electrolyte move in different directions because of regular dissemination. The distance is a few m to 10 m and doesn’t necessarily move straightly between terminals, which is one reason for the lessening in ionic conductivity. In the current review, in this way, the presentation of cross-connected stake-based strong polymer electrolytes was improved by compositing them with micron-sized permeable films.
This polymeric strong electrolyte shows superior execution as an electrolyte as well as being expected to be compelling in stopping the development of Li dendrites (dendritic gems), which can cause staining because of the consideration of a permeable layer. Through the acknowledgement of protected, superior execution LIBs, this accomplishment will add to the acknowledgment of a maintainable energy supply, which is the seventh objective of the SDGs.
The review was published in iScience.
More information: Manjit Singh Grewal et al, Increasing the ionic conductivity and lithium-ion transport of photo-cross-linked polymer with hexagonal arranged porous film hybrids, iScience (2022). DOI: 10.1016/j.isci.2022.104910
Journal information: iScience