Most scientists today figure out science through the standards of natural chemistry. Cells impart and enact processes through substance signals, and conventional medication has long centered around how to treat sickness by adjusting those signs.
However, a significant factor that influences cell behavior is overlooked by this method: physiology and anatomy.
According to Brenton Hoffman, Duke University’s James L. and Elizabeth M. Vincent Associate Professor of Biomedical Engineering, “applying physical force to a cell can alter and control the cell’s structure and behavior.” For instance, powers produced by streaming blood assist veins and corridors with developing into the right shape and acting ordinarily.”
However, abnormal behaviors associated with diseases like breast cancer can also be caused by altered mechanics.
“A breast tumor is typically identified by a hard lump that forces cells to produce additional mechanical force and may result in cellular migration. According to recent research, these bodily alterations might really be a catalyst for the growth of cancer.”
Brenton Hoffman, the James L. and Elizabeth M. Vincent Associate Professor of Biomedical Engineering at Duke University.
According to Hoffman, “cells generate more mechanical force, which can lead to cellular migration,” and “a breast tumor is usually discovered when someone feels a stiff lump.” Late work has shown that these actual changes could really be a trigger for disease to spread.”
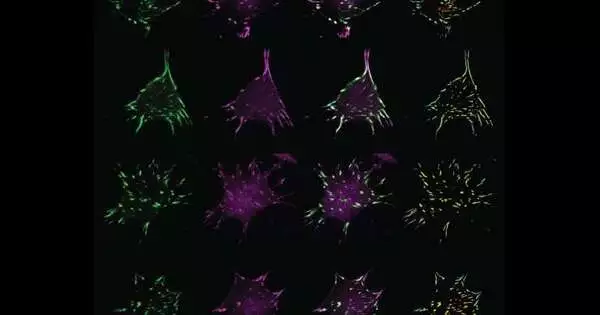
Mechanobiologists are still developing tools to answer fundamental questions about how various mechanical cues can disrupt cells and their signaling pathways because the field is relatively new. In a new paper that was published in Developmental Cell in March, Hoffman and his team described a novel imaging strategy for observing how forces in a living cell can affect a protein and its environment.
There were two primary approaches to studying the effects of mechanical forces on protein behavior prior to this work. The first method involves applying forces to purified and removed protein pairs, or individual proteins. While this can precisely determine whether force influences the behavior of the protein, it cannot investigate how these behaviors affect other proteins or the structure of the cell.
Catching proteins that leave cellular structures when these structures are forced to remain still is the alternative strategy. Although this method can identify a large number of proteins with some force sensitivity, it does not provide much information to scientists about how these proteins interact with one another and form complex structures.
Hoffman and his team developed FTC, or fluorescence-tension co-localization, to bridge the gap between these approaches. With FTC, researchers can examine how a protein’s local concentrations change when another protein is physically poked.
“FTC permits us, interestingly, to perceive what mechanical powers experienced by one protein mean for its capacity to draw in different proteins,” says Hoffman. “This is a significant achievement since modifying protein vicinity is one of the principal ways that flagging pathways get initiated, which controls cell conduct.”
As a proof of concept, Hoffman and his group considered the linker protein vinculin, which is found in sub-cell structures known as central grips that offer actual help to cells. The team was able to figure out how twenty other proteins moved in response to changes in the forces experienced by vinculin by imaging and analyzing more than 5,000 cells and more than 500,000 focal adhesions.
The team found that when force was applied, the majority of the proteins moved toward vinculin. However, they also found that the amount of force applied, the location of the force, and the maturity of the vinculin-containing focal adhesion all had an impact on which proteins were recruited by vinculin.
Tension, for instance, increased interactions with proteins that control adhesion to the support structure around the vinculin when it was part of an immature focal adhesion. In contrast, tension increased interactions with proteins that control the cell’s capacity to contract if vinculin was part of a mature focal adhesion. Mechanical stimuli likely have strong and diverse effects on cells because of this context dependence.
According to this work, Hoffman is central to propelling the area of mechanobiology, as recognizing these sorts of delicate protein connections will assist analysts with translating force-sensitive flagging pathways. Researchers may be able to stop the signaling that causes undesirable cell behaviors or even disease if they can learn how the environment affects vinculin’s interactions with other proteins.
“Many diseases, such as cancer, asthma, cardiovascular disease, and muscular dystrophy, do not have a chemical treatment option that is either safe or effective. According to Hoffman, these all clearly have a mechanical component, suggesting an untapped method for identifying novel therapeutic targets. The key to understanding these mechanical effects is providing the tools necessary to conduct these fundamental studies of how force affects the signals proteins send to their surroundings. This has the potential to influence fields as diverse as tissue engineering and medicine.”
More information: Arnold Tao et al, Identifying constitutive and context-specific molecular-tension-sensitive protein recruitment within focal adhesions, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.02.015