Polychlorinated biphenyls (PCBs) are widely used in modern and commercial products such as plastics, paints, electronic equipment, and protective liquids.Their assembling was widely prohibited from the last part of the 1970s on because of their harmfulness, yet huge sums actually stay in our current circumstance and amass inside creatures’ bodies.
Chiral PCBs will be PCBs that have two perfect representation isomers; these isomers are indistinguishable impressions of one another with a similar piece. Chiral PCBs are especially risky on the grounds that they have more chlorine iotas, which are difficult for the body to break down, so they can amass in the body effectively, and their isomers are used in an unexpected way, causing isomer-explicit harmfulness (especially neurodevelopmental issues). Nonetheless, the cycle behind this specific digestion was not recently known.
To address this, a group of researchers discovered how the body’s proteins use the perfect representation isomers unevenly.These findings will allow researchers to track PCB digestion and detoxification pathways in animals.They will also contribute to the advancement of innovation by forecasting the mirror isomers of chiral PCBs, allowing us to gain a better understanding of potential toxicity in humans and other vertebrates.
The examination results were distributed in natural science, innovation, and chemistry.
Despite the fact that the production and utilization of PCBs were prohibited a long time ago, they actually stay in the environment. PCBs have been discovered to accumulate inside groups of people and other creatures through food consumption.Specifically, PCBs with numerous chlorine bonds are water-safe and don’t separate without any problem.
This empowers large groups of these PCBs to amass inside creatures’ bodies, which negatively influences their wellbeing. The aryl hydrocarbon receptor (AhR) causes PCB toxicity, which has similar negative effects to dioxin toxicity, for example, disease, teratogenesis, and safe framework harm.Research is being led on the specific sorts of PCBs well known to cause these impacts, which are dioxin-like PCBs with one ortho-chlorine replacement in the biphenyl ring of their compound design or PCBs without any replacements.
In any case, if a PCB has multiple chlorine replacements at the ortho position of the biphenyl ring, it turns into a perfect representation isomer called a chiral PCB. These chiral PCBs don’t show dioxin-like harmfulness, yet they are undeniably more risky, restricting the ryanodine receptors (RyR) in creatures to become neurotoxic.
The two perfect representation isomers (called atropisomers) in chiral PCBs have indistinguishable physical and compound properties and exist at a 1:1 proportion in chiral PCBs, yet one-sided proportions are often seen in the climate and in creatures like worms and whales, as well as in people. It is accepted that this sort of uneven proportion is mostly brought about by digestion and that one of a chiral PCB’s atropisomers is more impacted by the metabolic response, hence lessening its focus.
As of recently, very little exploration has been done into the contrasts in how these atropisomers are used or the primary structure of the metabolic proteins.
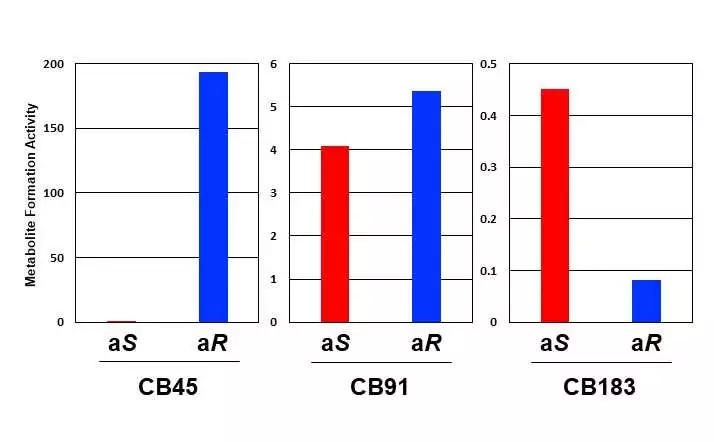
Human CYP proteins’ metabolite-development action against chiral PCB atropisomers: Of the two atropisomers in each chiral PCB, (aR)-CB45 in CB45 and (aS)-CB183 in CB183 are used more for hydroxylated metabolites. In CB91, each atropisomer is used at a comparable rate.
To address this information hole, the group led research focusing on the metabolic protein cytochrome P450 (CYP chemical). The CYP protein responds to unfamiliar mixtures that enter a creature’s body (for instance, synthetics or toxins in food or medications). CYP can change them into water-solvent mixtures and advance their removal from the body. Past exploration by this gathering has shown that CYP proteins hydroxylate and dechlorinate dioxin-like PCBs.
This reduces a PCB’s limiting with AhR and increases its water solubility, accelerating its removal from the body and thus reducing its toxicity.As such, CYP is a significant protein that determines if PCBs are treated as harmful mixtures by the body. To gauge the metabolic activity of CYP on chiral PCB, the scientists set up a CYP protein and PCB docking model. They utilized this to gauge the design of PCB metabolites and the construction of the CYP that chooses to diversely use every one of the PCB atropisomers.
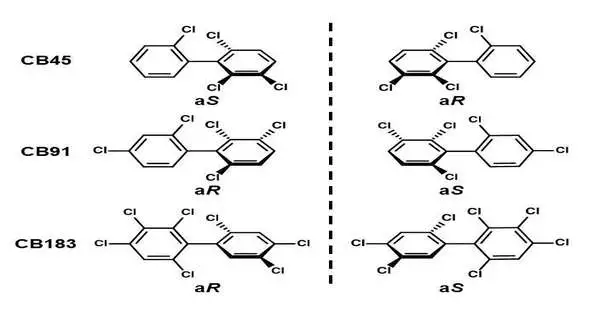
For the trial, the gathering has chosen three kinds of chiral PCB, each with an alternate number of subbed chlorine iotas: CB45 (4 chlorine replacements), CB91 (5 chlorine replacements), and CB183 (7 chlorine replacements). They used chromatography to isolate the atropisomers for each type of chiral PCB and allowed them to respond with a human CYP protein.It is assumed that research into isolating the atropisomers and allowing them to respond has not yet been completed.
The outcomes uncovered huge contrasts in how each atropisomer is used. Despite the fact that the two atropisomers on one PCB have similar physical and chemical properties, they are naturally unique. The analysts found that one of the chiral PCB atropisomers was used more than the other one, upsetting the 1:1 proportion.
Also, it is felt that the amount of (aS)-CB183 atropisomer diminishes on the grounds that it is used more than the other atropisomer, and this is upheld by the reports of low amassing of (aS)-CB183 in people.
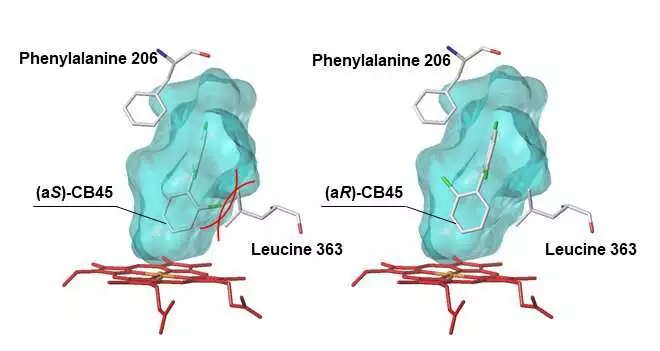
Human CYP protein docking model with the chiral PCB CB45:Light blue shows the substrate-restricting pit inside the CYP chemical. The CYP protein can’t frame a steady connection with the atropisomer (aS)-CB45 on the grounds that (aS)-CB45 impacts (shown by the red lines) leucine 363; hence, CYP produces fewer metabolites.
Yet, for what reason are these truly and artificially indistinguishable atropisomers used diversely by the CYP protein? To settle this secret, the scientists utilized a PC model to explore how effectively each chiral PCB atropisomer ties to the compound design of CYP. They tracked down that when an atropisomer topped off the substrate-restricting pit inside the CYP protein, CYP’s amino acids (that structure the pit) impeded the limiting interaction between CYP and the atropisomer.
Hence, the atropisomer that isn’t impeded by CYP’s amino acids turns out to be easy to use (atropisomer (aR)-CB45 in CB45 and (aS)-CB183 in CB183), bringing about changes to the first 1:1 proportion of atropisomers found in chiral PCB.
The consequences of this examination will be helpful for setting expectations about the atropisomers of chiral PCBs, which accumulate effectively inside creatures’ bodies. As such, it will be feasible to resolve which atropisomer is decreased by the metabolic response with CYP proteins and which atropisomer stays inside the body. The harmfulness of a chiral PCB is enacted by tying with RyR; however, the capacity to bind with RyR varies between the atropisomers. Hence, this exploration will make it conceivable to gauge the harmfulness of chiral PCBs.
More information: Hideyuki Inui et al, Differences in Enantioselective Hydroxylation of 2,2′,3,6-Tetrachlorobiphenyl (CB45) and 2,2′,3,4′,6-Pentachlorobiphenyl (CB91) by Human and Rat CYP2B Subfamilies, Environmental Science & Technology (2022). DOI: 10.1021/acs.est.2c01155
Terushi Ito et al, Enantioselective metabolism of chiral polychlorinated biphenyl 2,2′,3,4,4′,5′,6-Heptachlorobiphenyl (CB183) by human and rat CYP2B subfamilies, Chemosphere (2022). DOI: 10.1016/j.chemosphere.2022.136349
Journal information: Chemosphere , Environmental Science & Technology