In a meaningful step forward for levelheaded medication plans, a Texas A & M AgriLife group has depicted a few protein designs of a vital player in cell processes. The development could bring novel thoughts for therapies for illnesses like Alzheimer’s, AIDS, and others.
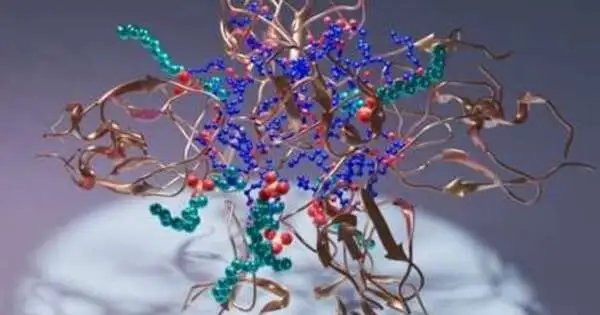
Imagine a protein kinase C C1 area (copper), its ligand diacylglycerol (blue), and a cleanser (cyan) being delivered creatively.politeness of Sachin Katti.
In particular, the work demonstrates the C1 area of protein kinase C, PKC, which directs the protein’s movement in living beings. In the designs, the C1 space folds over various particles of extraordinary remedial interest, giving the main dependable, nuclear goal guide for planning drug applicants.
“Protein kinase C is one of the most studied proteins in cell biology and pharmacology. A significant impediment has been a lack of precise structural knowledge to assist medication design attempts.”
The exploration was coordinated by Tatyana Igumenova, Ph.D., academic partner in the Department of Biochemistry and Biophysics in the Texas A&M College of Agriculture and Life Sciences. The undertaking’s essential creator is Sachin Katti, Ph.D., a postdoctoral individual working with Igumenova.
The review included a joint effort with Inna Krieger, Ph.D., research assistant teacher, and James Sacchettini, Ph.D., teacher, both in the Department of Biochemistry and Biophysics.
Awards from the National Institutes of Health and the Welch Foundation upheld the work.
One of the most sought-after protein structures
A sound cell answers synthetic signals in exact, mind-blowing ways. Getting compound contributions from the cell’s current circumstance and sending them to the focal control frameworks inside the cell core is the errand of particular proteins like PKC.
Ill-advised PKC action appears in numerous human illnesses. Accordingly, there is a lot of interest in tracking down ways of fine tuning PKC movement with drugs. The use of such medications will offer new methodologies for treating Alzheimer’s illness, AIDS, and malignant growth, and that’s just the beginning.
“Protein kinase C is one of the most strongly concentrated on proteins in cell science and pharmacology,” Igumenova said. “A significant obstacle has been the absence of exact primary data to direct medication plan endeavors.”
One point of entanglement for drug configuration is that the PKC family has 11 individuals. Different PKC relatives can have inverse physiological impacts, so an effective medication applicant should be specific about which PKC it targets.
To do that, drug competitors should fit an objective PKC like a key to a lock. Yet, deciding the 3D design of a PKC “on-switch”–the C1 area–bound to PKC activators has not been simple.
Utilizing X-beam crystallography, protein structures are commonly tackled. The method includes utilizing X-beams to determine the place of molecules in a gem. For this strategy to work, specialists need to create conditions where the protein of interest takes shape. However, extraordinary efforts in many examination labs in recent years have failed to yield precious stones of C1 spaces bound to relevant ligands.On account of this absence of progress, various analysts deemed the undertaking inconceivable, Igumenova said.
Tackling a 30-year issue
Precious stones of an area of protein kinase C were unexpectedly shaped in Katti’s NMR test tube. Sachin Katti’s civility as photographed.
Tolerating the issue as trying, Katti and Igumenova chose rather to concentrate on the atoms in the arrangement utilizing atomic attractive reverberation, NMR, and spectroscopy. This requires tracking down the right parts to copy cell films, where the C1 area would experience ligands.
Then, one fine day, Sachin discovered gems shaping in an old NMR tube,” Igumenova explained.”I give all the credit to Sachin, who essentially said, ‘I will proceed to test them and check whether they are really the protein.’ And he was correct. It gave us certainty that crystallization is conceivable.
Thus, Katti gives trustworthiness to the experiences acquired from NMR and a touch of karma.
“I imagine that is the excellence of doing research where you need to utilize numerous methodologies,” he said. “No one can really tell when one methodology will be valuable for accomplishing something with different methodologies.”
Experiences from NMR and X-beam crystallography
The new protein structures, alongside the group’s NMR results, have proactively yielded fascinating data. One well-established secret has been the means by which C1 areas can oblige ligands that have altogether different synthetic designs, Igumenova said.
“Our past NMR work demonstrated that the circles of the C1 space that tight spot ligands occupy are extremely unique,” Igumenova said. This C1 area resembles a PAC-man. It ties the layer, and afterward it looks for a ligand. When it finds the ligand, it hooks on. “
Furthermore, the design shows that the ligand-restricting section has a “water-adoring,” or hydrophilic, surface at the base and a “water-repulsing,” or hydrophobic, surface at the top.
“Assuming that you contemplate a lipid particle, the head bunch is hydrophilic and the tail is hydrophobic,” Igumenova said. “In this way, when C1 spaces tie lipid ligands, the examples match.”
The group’s outcomes incorporate the design of a C1 space bound to its normal ligand, diacylglycerol. Furthermore, the group demonstrates a few different designs of C1 that incorporate various builds of pharmacological interest.
The work additionally gives a strategy to test different medication competitors, Katti said.
“If you have any desire to concentrate on fish, you need to concentrate on them in water,” Katti said. “Presently, we know how to establish a layer like climate where these exceptionally hydrophobic mixtures can be tried for C1 restricting.”
Then, Katti and Igumenova plan to investigate C1 areas with other PKC relatives.
“We must zero in on C1 spaces since they have intrinsic contrasts that can be taken advantage of to accomplish selectivity,” Igumenova said. “What we are finding currently is that not all C1 spaces are made equivalent.”
Story Source:
Materials given by Texas A & M AgriLife Communications Unique, composed by Olga Kuchment. Note: Content might be altered for style and length.