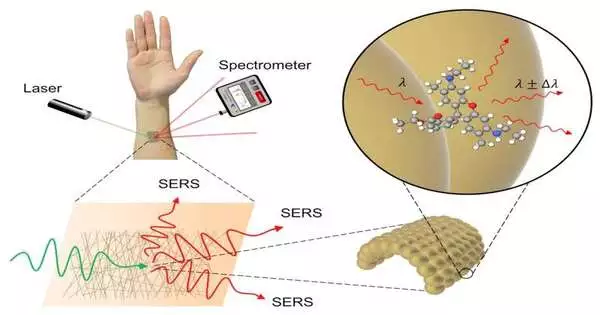
Scientists made an exceptionally ultrathin sensor, made from gold, that can be joined straightforwardly to the skin without bothering or inconvenience. The sensor can quantify different biomarkers or substances to perform on-body compound investigation. It works by utilizing a strategy called Raman spectroscopy, where laser light focused on the sensor is changed somewhat depending upon what synthetics are available on the skin by then. The sensor can be finely tuned to be very delicate and is powerful enough for pragmatic use.
Wearable innovation is the same old thing. Maybe you or somebody you know wears a smartwatch. Large numbers of these can screen specific wellbeing matters, for example, pulse, but at present they can’t gauge synthetic marks, which could be valuable for clinical conclusion. Smartwatches or more specific clinical screens are also moderately cumbersome and frequently very expensive.Provoked by such deficiencies, a group of scientists from the Department of Chemistry at the University of Tokyo looked for a better approach to detect different medical issues and ecological issues in a painless and savvy way.
“There is also a chance that the sensor will work with techniques for chemical analysis outside Raman spectroscopy, such electrochemical analysis, but all of these concepts need to be thoroughly investigated.”
Professor Keisuke Goda
“A couple of years prior, I ran over a captivating strategy for creating vigorous stretchable electronic parts from another examination bunch at the University of Tokyo,” said Limei Liu, a meeting researcher at the time of the review and as of now a speaker at Yangzhou University in China. “These gadgets are turned from ultrafine strings covered with gold, so they can be appended to the skin without issue as gold doesn’t respond to or bother the skin in any capacity. As sensors, they were restricted to recognizing movement, notwithstanding, and we were searching for something that could detect synthetic marks, biomarkers, and medications. So we based upon this thought and made a painless sensor that surpassed our assumptions and propelled us to investigate ways of further developing its usefulness much further. “
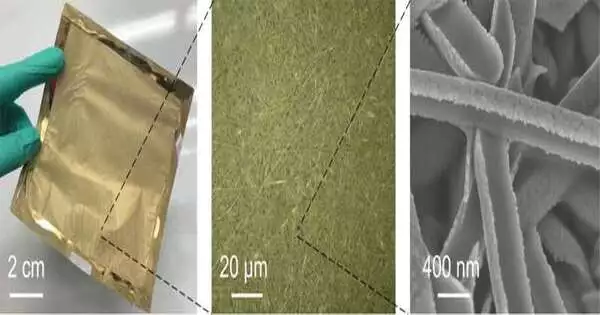
The gold nanomesh at various amplifications The singular strands are around one-five-hundredth the thickness of human hair. Goda and colleagues
The principal part of the sensor is the fine gold lattice, as gold is lifeless, implying that when it comes into contact with a substance the group wishes to gauge — for instance, a potential sickness biomarker present in sweat — it doesn’t synthetically modify that substance. In any case, all things being equal, as the gold lattice is so fine, it can give a shockingly enormous surface for that biomarker to tie to, and this is where different parts of the sensor come in.
As a low-power laser is pointed at the gold cross section, a portion of the laser light is ingested and some is reflected. Of the light reflected, most has the same energy as the approaching light. Be that as it may, some approaching light loses energy to the biomarker or other quantifiable substance, and the disparity in energy between mirrored and incident light is novel to the substance being referred to. A sensor called a spectrometer can utilize this extraordinary energy fingerprint impression to distinguish the substance. This strategy for substance ID is known as Raman spectroscopy.

“Right now, our sensors should be finely tuned to identify explicit substances, and we wish to push both the awareness and particularity significantly further in the future,” said Assistant Professor Tinghui Xiao. “With this, we think applications like glucose observing, ideal for victims of diabetes, or even infection identification, may be conceivable.”
“There is also potential for the sensor to work with different techniques for synthetic examination other than Raman spectroscopy, like electrochemical investigation, yet this multitude of thoughts requires significantly more examination,” said Professor Keisuke Goda. “Regardless, I trust this examination can prompt another age of minimal-expense biosensors that can alter wellness checking and diminish the monetary weight of medical care.”
More information: Limei Liu et al, Highly Scalable, Wearable Surface‐Enhanced Raman Spectroscopy, Advanced Optical Materials (2022). DOI: 10.1002/adom.202200054