Couldn’t it be a rich answer to utilize the substance that is generally harmful to the environment and compromises the future as a natural substance for financial products and ordinary things? As a matter of fact, carbon dioxide (CO2), an undeniable result of development, is now being utilized in the research facility to create lower olefins, alcohols, and fills in mix with hydrogen and other substance reactants, which can all be gotten economically. For such cycles to become a modern practice, they should have the option to run under “fluctuating” conditions.
“Fluctuating” implies that these cycles should likewise work with fluctuating supplies of energy and beginning materials. This is new for science, yet it can’t be kept away from if the energy, for instance, for the creation of hydrogen by electrolysis, is to come from sustainable sources like the sun and wind from now on. These are not accessible consistently or by any stretch of the imagination, on windless evenings.
“However, we prefer to obtain methane from it. Methane, with the chemical formula CH4, is the major component of natural gas and the most basic hydrocarbon. However, we are looking for higher-value hydrocarbons, such as olefins right now. These are essential fundamental compounds that can be refined chemically.”
Prof. Dr. Evgenii Kondratenko at the Rostock Leibniz Institute for Catalysis explain.
Notwithstanding, the impact of dynamic circumstances on compound responses has scarcely been concentrated up to this point, as Prof. Dr. Angelika Brückner and Prof. Dr. Evgenii Kondratenko at the Rostock Leibniz Foundation for Catalysis make sense of. Their examination group is associated with work on creating impetuses for CO2 hydrogenation of higher hydrocarbons under fluctuating circumstances.
From environmental gases to unrefined substances
When it’s all said and done, the objective is to substitute oil as a natural substance base and simultaneously convert a hazardous waste gas, carbon dioxide, into a natural substance. Yet, CO2 is an inactive gas and barely responds all by itself.
To acquire economically helpful mixtures, particularly essential materials for the synthetic blend of important items, the very steady carbon-oxygen bonds in CO2 should be enacted. This is done chemically by hydrogenation, i.e., hydrogen is added, which can create a wide range of hydrocarbon compounds. The more carbon iotas they contain in the atom, the higher their valence.
For the cycle, the scientists changed the supposed Fischer-Tropsch union, which was grown very nearly a long time ago for the hydrogenation of carbon monoxide (CO). To do this, they replaced the exceptionally harmful CO with CO2.
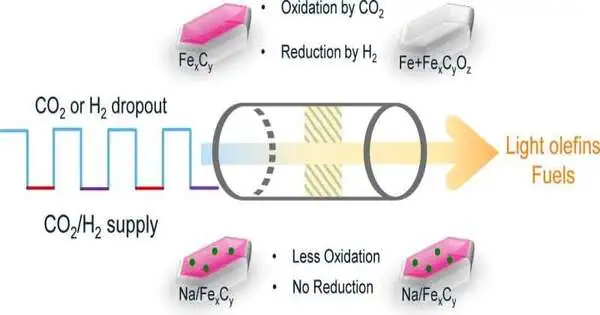
Iron impetus changes
The design and properties of the impetus assume a key role in figuring out which items are shaped during CO2 hydrogenation. According to Brückner and Kondratenko, it is an iron impetus. However, not all iron is very similar, they say. They found that the impetus changes in the response under fluctuating circumstances: it continues to shape new “stages and” in the middle between, they say.
To definitively recognize good and obstructive stages, the scientists notice the impetus at work, as it were. This is finished by utilizing profoundly unambiguous operando-spectroscopic investigation strategies in view of infrared, UV, and laser light. The impetus tests were halfway evolved by the actual analysts and somewhat gotten from their exploration accomplice at Humboldt College in Berlin.
Prof. Kondratenko explains that iron is frequently used chemically as an oxide. “However, we like to acquire methane with it.” With the sub-atomic equation CH4, methane is the fundamental part of flammable gas and the easiest hydrocarbon. “Nonetheless, we are after higher-esteem hydrocarbons, right now, for instance, olefins.” These are vital fundamental synthetics that can be additionally refined synthetically.
Dynamic synergist stage: iron carbide
The Rostock researchers found that one stage specifically is pivotal for CO2 hydrogenation, in which iron carbide is shaped at the impetus surface. Furthermore, they figured out how to settle this carbide stage and keep away from the meddling stages. For instance, by utilizing iron oxalate, an iron salt of oxalic corrosive (FeC2O4), rather than the typical iron oxide as the impetus material, As of late, the group has distributed two articles on this point in Catalysis Science and Innovation and The Diary of Catalysis.
A third distribution of the gathering, in ACS Catalysis, covers the issue of the diminishing action of the iron impetus. As a result, the Rostock scientists found intermediates who believed in coke in specific situations. This is kept as an intense layer on the impetus surface and clouds the dynamic species.
For future plants, the vision is to convert the carbon dioxide right where it gathers as a huge mob and use it as an unrefined substance, say Prof. Dr. Brückner and Prof. Dr. Kondratenko. As per the standards of green science and the roundabout economy, such a cycle would be especially reasonable in the climate of the right now biggest CO2 producers, like the concrete business, iron and steel creation, and the substance business itself, where the CO2 can be additionally handled with “green” hydrogen into significant hydrocarbons.
More information: Qingxin Yang et al, The role of Na for efficient CO2 hydrogenation to higher hydrocarbons over Fe-based catalysts under externally forced dynamic conditions, Journal of Catalysis (2023). DOI: 10.1016/j.jcat.2023.07.012
Andrey S. Skrypnik et al, Spatial analysis of CO2 hydrogenation to higher hydrocarbons over alkali-metal promoted iron(ii)oxalate-derived catalysts, Catalysis Science & Technology (2023). DOI: 10.1039/D3CY00143A