Subbed aromatics are among the main building blocks for natural mixtures, for example, drugs, crop-safeguarding specialists, and numerous materials. The capability of the replacement design is hampered by the spatial game plan of the different structure.
An Otto Diels Foundation of Natural Science exploration group at Kiel College has now introduced a strategy in the Chem diary to create compounds with an especially appealing structure, but to test them on a regular basis to get to a replacement design more effectively than previously. To empower the necessary initiation of carbon-hydrogen (C-H) bonds, they fostered a unique palladium impetus that can, interestingly, specifically move toward a formerly unthinkable situation inside particles.
Shutting a long-lasting examination hole
With their new strategy, the researchers close a long-term research hole.
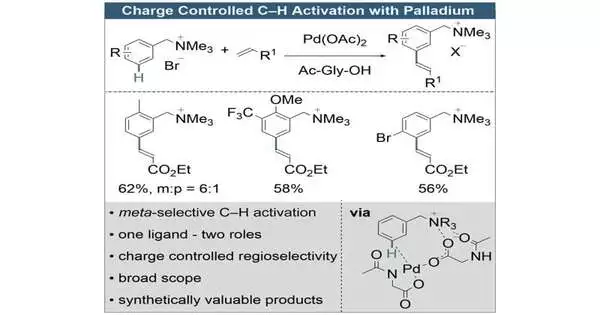
“On a fundamental level, subbed sweet-smelling compounds have three situations to which an impetus can be added to instigate a response—ccalled ortho, meta, and para.” “Contingent upon the position, different compound items with generally various properties are framed toward the end,” says Manuel van Gemmeren, Teacher of Natural Science at Kiel College.
“In theory, substituted aromatic compounds have three places where a catalyst can attach to produce a reaction: ortho, meta, and para. At the end, depending on the position, multiple chemical compounds with fundamentally different features are created.”
Manuel van Gemmeren, Professor of Organic Chemistry at Kiel University.
It is now known how to explicitly allow impetuses to go after the ortho and para positions.Currently, Manuel van Gemmeren and his team can also specifically focus on the meta position.This permits them to deliver meta-substituted benzyl ammonium species, which are adaptable mixtures for additional elaboration in natural science.
Ordinarily, these mixtures just show up in limited quantities blended in with different items. “As of recently, they must have been isolated from one another with a great deal of exertion.” On the other hand, you wanted dreary, engineered courses to be created in a designated way. The two cases “brought about superfluous side effects,” which makes sense to van Gemmeren.
With the new technique, meta-substituted benzyl ammonium mixtures can now be delivered substantially more productively. The examination group around van Gemmeren utilized a rule that had not been depicted in that frame of mind previously: the palladium impetus they planned can collaborate with charges in the particle.
This drastically alters the creation of subsequent items for the previously difficult-to-deliver replacement design.Calculations by partners at the Catalan Institute of Substance Exploration (ICIQ), Spain, revealed that charge collaborations are unquestionably to blame.
Strategy is also appealing to pharmaceutical and agricultural companies.
These discoveries from primary research can also be useful for pharmaceutical or farming organizations that create massive libraries of primarily related particles to focus on their natural action.” Any place the biggest conceivable assortment of mixtures is deliberately inspected, our strategy can be a useful device to close past information holes,” says van Gemmeren.
The development of the new technique is the result of numerous long stretches of preliminary work that began at the University of Münster. Here van Gemmeren set up his own exploration group on the actuation of C-H bonds through the Emmy Noether program of the German Exploration Establishment (DFG) before he came to Kiel College in 2022. In Kiel, he will likewise carry out his ERC Beginning Award project, “DULICAT,” from which the calculated thought for the new technique arose.
More information: Arup Mondal et al, Charge-controlled Pd catalysis enables the meta-C–H activation and olefination of arenes, Chem (2023). DOI: 10.1016/j.chempr.2022.12.019
Journal information: Chem