A gathering led by Prof. Horacio Cabral, as a team with a gathering led by Prof. Kazuhiro Kakimi, found a better approach to further developing disease immunotherapy. Their discoveries are distributed in a paper, “An IL-12-Based Nanocytokine Securely Potentiates Anticancer Resistance through Spatiotemporal Control of Irritation to Kill Progressed Cold Cancers,” in Cutting Edge Science.
Many aggressive cancers have an immunosuppressive microenvironment that prevents safe cell invasion, activation, and effector capability, resulting in an immunologically cool growth aggregate that is resistant to immunotherapy led by resistant designated spot inhibitors (ICIs), including the popular anti-PD-1 and anti-CTLA-4 antibodies.
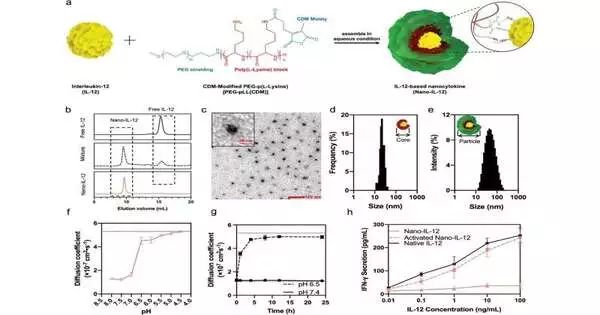
Interleukin-12 (IL-12) is one of the most stable and beneficial cytokines.Hence, there is a significant premium to utilize it for upgrading the resistance in cancers and further developing the reaction rates of immunotherapies. In any case, IL-12 is very harmful because of its fundamentally resistant actuation and shows restricted clinical adequacy under the safe portion. To tackle such an issue, an IL-12-based nanocytokine that has some control over the safe enactment capability of IL-12 in view of intratumoral pH was created to understand a growth-designated resistance upgrade.
Because of the pH-sensitive polymer materials, the nanocytokine can detect the pH in growths and release the fully dynamic cytokine.As a result, the nanocytokine avoids the fundamental reaction mediated by IL-12 and reduces the aftereffects.In the murine triple-negative bosom disease (TNBC) model, the nanocytokine upgrades the resistance and lifts the safe cells’ penetration level in the growth. The nanocytokine likewise mixes power with ICIs, totally killing both essential and metastatic cancers.
What is the oddity of this review?
IL-12 has long been investigated as a potential enemy of disease specialists, yet its solid harmfulness and lack of helpful adequacy in the most endured portion were demonstrated by early phase clinical preliminary results, necessitating systems to address such issues before its application. The nanocytokine plan is the main illustration of a polymer-based switchable IL-12 conveyance framework, and in the consequences of this review, it was seen as:
- A polymer-based nanocytokine plan can steadily control the “off” and “on” provinces of IL-12’s bioactivity by detecting the pH contrast between solid organs and growth.
- The cancer-related activation of the nanocytokine prompts spatiotemporal control of the IL-12-mediated safe responses. In solid tissues, the off-target safe actuation and balancing responses are reduced, and in the mean time, during growth, the fiery reactions are improved.
- The nanocytokine significantly stimulates cold growths at the quality articulation level and synergizes with ICIs to completely kill advanced essential and metastatic cancers.
For what reason are these discoveries significant, and how is everything working out with the ongoing treatment?
Interleukin-12 (IL-12) is one of the most stable and beneficial cytokines. Hence, there is a significant premium to involving it in the kindling of cancers and expanding the response rates of immunotherapies. IL-12, by the way, is very dangerous because of its fundamental safe enactment and has limited clinical adequacy, even at its most endured portion (MTD), because its spatiotemporal fiery elements trigger checking resistant responses.
While significant efforts are being devoted to designing the IL-12 protein in order to expand its security and cancer selectivity, these methodologies fall short of addressing IL-12’s issues because they may still cause fundamental irritation, advance meddling calming signals, and, on average, have lower equimolar action than local IL-12.Sadly, the fiery elements of such protein-designed frameworks are still inadequately perceived, and the advancement of balancing optional reactions remains hazy.
The IL-12-based nanocytokine immediately addresses the expressed issues by advancing the spatiotemporal control of the irritation via high cancer accumulation, high and specific intratumoral safe action, and managed connection with the fundamental resistance. Hence, nanocytokines control irritation in a spatiotemporal way.
It slowed the basic safe reaction and prevented the emergence of versatile calming signals after repeated infusion, for example, the extension of interleukin-10 (IL-10), which is normally seen after the second infusion of IL-12 or standard IL-12-based frameworks, for example, combination proteins and immunocytokines. Such spatiotemporal control may be advantageous for acknowledging rehashed organizational plans while minimizing counter-growth effects.
The nanocytokine likewise extended the helpful window of IL-12 through the spatiotemporal control of irritation. As a result, the nanocytokine took advantage of the impediment to balancing safe reactions to reduce the viable portion against immunosuppressive triple-negative bosom disease by 10%.Besides, the nanocytokine upgraded the security and was non-harmful even at a portion that is 1,000 fold higher than the revealed MTD of local IL-12 in human examinations.
The nanocytokine significantly induced immunosuppressive growths, preventing feelings of safety that impede adequacy. This permitted the nanocytokine to firmly synergize with safe designated spot inhibitors (ICIs) by switching the immunosuppressive cancer microenvironment.
On the grounds that the nanocytokine depends on biocompatible, FDA-endorsed materials, the system holds high potential for clinical interpretation. Likewise, the plan could be extended to other helpful proteins, filling in as a viable system for growth and designated conveyance of biologics.
More information: Pengwen Chen et al, An IL‐12‐Based Nanocytokine Safely Potentiates Anticancer Immunity through Spatiotemporal Control of Inflammation to Eradicate Advanced Cold Tumors, Advanced Science (2023). DOI: 10.1002/advs.202205139