The University of Illinois’ plasma engineers and chemists have demonstrated a cost-effective, rare metal-free method for forming carbon-carbon bonds—the foundation of all organic compounds—without the need for costly catalysts.
Researchers in Nuclear, Plasma, and Radiological Engineering, Bioengineering, and Chemistry at the University of Illinois pooled their expertise to create this novel metal-catalyst-free strategy that, the researchers claim, has the potential to alter the course of organic chemistry.
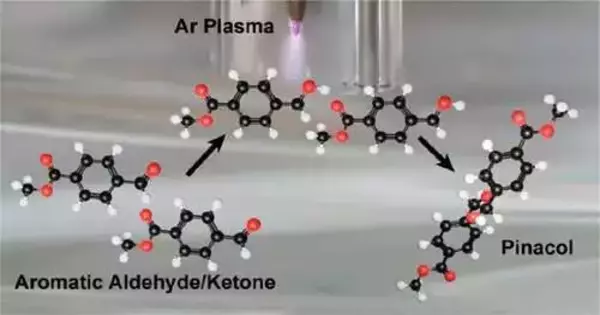
In the review, “Plasma Electrochemistry for Carbon Security Development through Pinacol Coupling,” distributed in the Diary of the American Compound Society, the group makes sense of how they utilized power and a plasma-fluid cycle to create solvated electrons to frame carbon bonds in a pinacol coupling response. The formation of C-C bonds is extensively utilized in the production of numerous synthetic chemicals, including plastics and pharmaceuticals.
Researchers say that this is the first time plasma-generated solvated electrons have been used in an organic redox coupling reaction and provides a long-term solution for other organic reductive reactions. Metal reagents or catalysts are typically required for these reactions, which are not only hard to come by and expensive but also pose safety or environmental concerns and sometimes necessitate the use of heat in the reactive process.
“Our process really only requires electricity—aside from the reactor cell and equipment—and hopefully, in the future, this can come from renewable sources like wind, solar, or nuclear, so the whole process is sustainable,”
Co-author R. Mohan Sankaran, Donald Biggar Willett Professor in Engineering in the Department of Nuclear, Plasma, and Radiological Engineering.
R. Mohan Sankaran, co-author of the study and Donald Biggar Willett Professor in Engineering in the Department of Nuclear, Plasma, and Radiological Engineering, stated, “Our process only really requires electricity—other than the reactor cell and equipment—and in the future hopefully this can come from renewable sources like wind, solar, or nuclear, so the whole process is sustainable.”
According to Sankaran, their method uses argon gas to produce electrons and then injects these electrons into a solution to create solvated electrons, a powerful chemical species typically produced through complex, equipment-intensive radiolysis.
“In our case, the solvated electrons are generated with just a DC power supply and a relatively simple electrolysis reactor that houses our electrodes and the solution where we have the organic substrates,” Sankaran said. His group has been developing atmospheric-pressure plasmas for more than a decade. In previous work, this type of plasma-liquid process has been applied to other applications such as the synthesis of nanoparticles and the fixation of nitrogen. We were interested in natural science, yet we had no skill in either the techniques or the portrayal.”
Sankaran, who contacted Jeffrey S. Moore, a research teacher in science, for mastery, said this undertaking could never have been conceivable without the coordinated effort.
“There is no way we would have succeeded without having someone with the requisite chemistry background,” Sankaran stated. “Most of this is chemistry—something that my group does not do.”
Jian Wang, the study’s lead author and postdoctoral associate, brought his knowledge of materials science and chemistry to the project. He collaborated with plasma expert Scott Dubowsky, the study’s co-author and research scientist in the Sankaran group, to learn about the plasma-liquid process and find an organic reaction to study.
Wang tried a variety of organic substrates and characterized reactions using a variety of analytical methods. In the end, they settled on pinacol coupling because it is a well-known reaction that forms carbon-carbon bonds and they thought it could work with the plasma liquid process. Matthew Confer, a postdoctoral researcher and another co-author in the group of Rohit Bhargava, professor of bioengineering and faculty affiliate in chemistry, used his expertise in computational chemistry to model the solvated electron chemistry and radical reactions that led to the formation of the pinacol product.
“This is a great delineation of the brilliant rule for fruitful cooperation: According to Moore, a Howard Hughes Medical Institute Professor, Professor Emeritus of Chemistry, and Stanley O. Ikenberry Research Professor, “the best collaborators share a common goal but bring different expertise.”
There have been a couple of studies where plasma was utilized for a natural response, which Sankaran made sense of, yet the responses and the system were unique.
“The plasma was normally used to oxidize a synthetic, and the compound that was delivered by the plasma was a responsive oxygen animal category.” Sankaran stated, “The reduction reaction, which required electrons (or solvated electrons), was the one we studied in our case. The reduction resulted in the formation of new carbon-carbon bonds.”
The next thing they’ll do is show that their method is universal and can be used for a variety of reactions by applying it to another organic chemistry reaction.
Sankaran said, “We also hope to find a reaction that is difficult to carry out because it has a low yield, requires harsh conditions, or there is no active metal.” He also said that they hope to address a problem that their study revealed, which is that yields can be limited by mass transfer limitations. Our response happens at the connection point between a plasma and the arrangement, and for the substrate to arrive at the connection point, it should diffuse. We can resolve this issue by integrating a fluid stream, which will upgrade mass vehicles by convection. We might also be able to scale up the process so that we can continuously produce a product with liquid flow.”
The high energy of plasma, according to Wang, presents a particular challenge for plasma electrochemistry as an alternative to more conventional organic synthesis.
“Despite the fact that we can accomplish moderately great yield and selectivity, the control is as yet not comparable to, for instance, conventional sciences with metal impetuses or electro- or photocatalytic sciences,” Wang said. “We are dealing with working on controllability and selectivity at the present time.”
More information: Jian Wang et al, Plasma Electrochemistry for Carbon–Carbon Bond Formation via Pinacol Coupling, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c01779