According to the viewpoint of physicists, pleurotin is a charming atom.
There is solid proof of undiscovered helpful properties as a growth inhibitor and anti-toxin. It has an entrancing, complex design (six rings! (Eight stereocenters!). Also, blending throughout the long term has been troublesome. The last time scientists pulled that off was in 1988, and they required 26 stages in order to make it happen.
For Princeton Chemistry’s Sorensen Lab, those characteristics were essential for the fascination for a drawn-out venture of significant investment that has happened as expected.
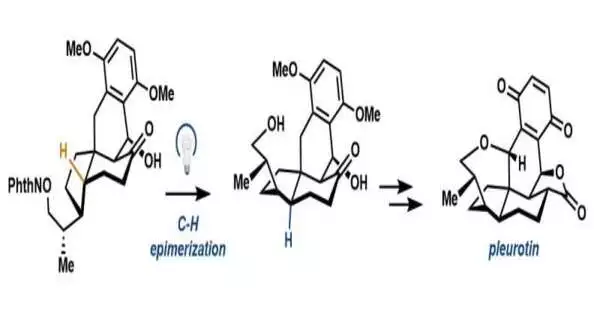
The lab reports a brief blend of pleurotin via the Diels-Alder response and an extreme epimerization that flips a cis-hydrindane to the ideal trans-hydrindane. Their late-stage middle encounters the achievement 1988 union near the end of the cycle, reducing the total number of steps required for the blend by thirteen.
The lab’s cycle could yield an extended group of pleurotin-like anticancer screening competitors, which, down the line, might be helpful to drug organizations hoping to take advantage of the commitment of pleurotin as a cutting-edge drug.
“Pleurotin is a very sensitive and reactive chemical. However, it has yet to be approved as a medicine, owing in part to its inability to dissolve in water. Ideally, you’d like to change its structure: adjust here, change there, add a hydroxy here or a phosphate there, and make some really cautious changes.”
John Hoskin
“Pleurotin is a touchy particle; it’s receptive. Yet, it hasn’t worked out as a medication yet, mostly on the grounds that it’s not very water-solvent, “said third-year graduate understudy John Hoskin, lead creator of the paper. “Preferably you need to modify its design: change here, change here, put a hydroxy here or a phosphate there, make a few cautious changes.”
“What’s more, since you can’t actually do that beginning from pleurotin itself, our methodology will be to integrate the progressions from a ground-up union, which is just conceivable due to the curtness of the course.” Then you end up with alleged analogs that are basically the same as this normal item yet have these essential changes. “
“A Concise Synthesis of Pleurotin Enabled by a Nontraditional C-H Epimerization” was published last month in the Journal of the American Chemical Society by Hoskin and P.I. Erik Sorensen, the Arthur Allan Patchett Professor in Organic Chemistry in the Department of Chemistry.
“At the point when a scientist takes a gander at a design like this one, there aren’t any undeniable systems that one ought to take to make it from basic mixtures,” said Sorensen. His lab initially started dealing with pleurotin in 2008, only to meet with a progression of frustrations. As of recently,
“Assuming you take pleurotin and say, I believe we should do site-specific science on its fringe so we could assemble new atoms with further developed properties, then maybe there will be better enemies of disease specialists,” he added. So John and I were attracted to the test of fostering a compound methodology for building that system in as few steps as could be expected.
“Eight stages is a small number of stages for a particle of that intricacy,” said Sorensen. “This exploration is a declaration of John’s expertise as a creator and agent of natural union.”
Beginning around 1947, an undiscovered commitment was made.
Pleurotin is derived from the Pleurotus griseus plant.Scientists originally depicted the particle in a paper distributed in 1947 as hindering the development of Staphylococcus aureus, the wellspring of bacterial sickness. That was 41 years before the achievement blend of pleurotin by David Hart, presently an emeritus teacher at Ohio State University.
But since the failure to blend it effectively, pleurotin has not been explored to its maximum capacity. That is the point at which the Sorensen Lab stepped in.
To pare down the means toward blend, scientists involved a proven strategy in natural union called a 1,5-hydrogen iota move, in which a receptive, oxygen-focused extremist basically “comes to over” and culls a hydrogen off a carbon that is essential for the pleurotin design to make another revolutionary. Scientists then utilized that extremist to get hydrogen from an exogenous thiol that would consider the stereocenter to flip to another option — or, trans — setup.
“We attempted many systems. What wound up working was this reversal move toward going from this cis-hydrindane to trans-hydrindane. That is the key knowledge, “said Hoskin. By using usefulness innate in the particle—this oxygen—we could, as though we were utilizing a tiny set of tweezers, pluck off this hydrogen and flip that carbon to get the required trans-hydrindane.
The cycle creates a racemic final result, making both left-and right-hand forms to equivalent extents. Only one of them is probably going to be bioactive. Since the proper blend has been finished in a more brief style, Hoskin expressed, the following test will create only one perfect representation form of the particle and analogs thereof.
“This examination shows the force of a short blend,” said Hoskin. “It just requires seven days to push through the entire course.”
Sorensen added: “I think this work sets us in a good situation toward our more extensive goal of growing the class of pleurotin-based anticancer specialists.”
More information: Kuo Zhao et al, Contra-Thermodynamic Positional Isomerization of Olefins, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c11681
Journal information: Journal of the American Chemical Society