Strong-state lithium-particle batteries rely upon the development of particles (charged molecules) in the strong, as opposed to fluid, state to charge or release the battery. These strong-state electrolytes are more secure, more cost-effective, and fit for higher energy densities than batteries that depend on fluid electrolyte arrangements, yet they experience the ill effects of low ionic conductivity, the development of particles, and unfortunate warm solidity.
Another composite strong electrolyte (CSE) film has been blended by specialists from Upper East Ordinary College utilizing lithium salts and an ionic fluid to work on the separation, and thus conductivity, of charged lithium molecules in a strong state electrolyte battery. The group distributed their outcomes in the diary Polyoxometalates on September 28, 2023.
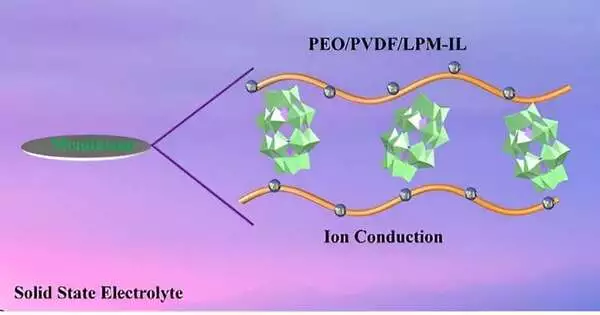
Polyoxometalates (POMs) are bunches of metal and oxygen molecules with properties not entirely set in stone by the obvious construction of the POM iota group. The scientists as of late presented a POM-based lithium salt, Li6P2Mo18O62 (LPM), into a strong polymer electrolyte (SPE) comprised of the polymer polyethylene oxide (PEO), an economical and stable chain of numerous ethylene oxide subunits.
“In contrast, inorganic solid electrolytes (ISEs) such as LPM… have high ionic conductivity. We harness their individual features… to obtain optimum mechanical properties and increase their ionic conductivity by combining ISEs like LPM into SPEs to build composite polymer electrolytes.”
Hong-Ying Zang, senior author of the paper and professor at Northeast Normal University in Changchun, China.
PEO experiences low ionic conductivity, and the expansion of LPM salt changes the properties of the polymer and upgrades particle development. The exploration group likewise consolidated an ionic fluid (IL) to liberate lithium particles from LPM, further working on the conductivity of the composite electrolyte material.
“Strong state electrolytes (SSEs) are thought of as the most encouraging contender for cutting-edge energy capacity gadgets because of their magnificent warmth and electrochemical soundness. Despite the fact that SPEs have astounding adaptability and thickness, they are seriously restricted because of their low ionic conductivity, poor mechanical strength, and low warm dependability at room temperature.
“Conversely, inorganic strong electrolytes (ISEs) like LPM normally have high ionic conductivity. By integrating ISEs like LPM into SPEs to frame composite polymer electrolytes, we influence their particular properties… to accomplish upgraded mechanical properties and work on their ionic conductivity,” said Hong-Ying Zang, senior creator of the paper and teacher in the Critical Research Center of Polyoxometalate and Reticular Material Science at Upper East Ordinary College in Changchun, China.
“At present, inorganic electrolyte fillers incorporate nanoparticles and ionic-conductive inorganics. As a class of metal-oxygen bunches, the utilization of polyoxometalates in strong-state batteries is hampered by the trouble of moving lithium particles. In this paper, we advance the separation of lithium particles from polyoxometalates with ILs. to grant LPM and IL composites (LPM-IL) great electrical conductivity,” said Zang.
The group described the particle conductivity and portability of the composite film by estimating the air conditioner impedance, or the opposition of the current stream in a circuit. The group found that electrolyte films containing the ideal convergence of LPM and IL exhibited multiple times higher conductivity than layers without IL.
Likewise, the group established that the conductivity of composite layers produced with polyvinylidene fluoride (PVDF), a non-responsive thermoplastic filler material, related to PEO expanded conductivity multiple times when contrasted with LPM-IL films orchestrated without PVDF. The composite film likewise showed great solidity for more than 12 hours at a temperature of 80 °C.
“The consequences of these analyses exhibit that polyoxometalates can be utilized as inorganic strong electrolytes,” said Zang. IL actually expanded the separation of lithium particles from LPM and worked on the ionic conductivity of the composite strong electrolyte film. The fuse of PVDF likewise made a PEO-PVDF conductive organization in the film that further advanced lithium particle development, improving conductivity.
The examination group accepts their special, PEO-based composite film containing PVDF, POM-based lithium salt, and IL, which gives a viable method for expanding ionic conductivity in strong-state electrolytes for use in lithium-particle batteries. “Our subsequent stage is to work on the exhibition of polyoxometalates to make better strong-state lithium-particle batteries,” said Zang.
More information: Qianqian Liu et al, Ionic liquid-mediated PEO-based solid-state electrolyte membrane modified with Dawson-type polyoxometalates, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140036