A review led by UT Southwestern researchers recommends that prostate disease cells be prevented from transforming into other cell types to beat the opposition they create to broadly endorsed treatments. The discoveries, published in Nature Malignant Growth, could prompt another way to deal with and battle this lethal illness, the second-most common disease in American men that kills almost 35,000 every year in the U.S.
“We figure this original mix treatment could decisively work on clinical results of prostate disease patients and ideally save many lives,” said concentrate on pioneer Ping Mu, Ph.D., right-hand teacher of sub-atomic science and an individual from the Harold C. Simmons Extensive Malignant Growth Center.
Drugs that focus on the androgen receptor (AR), a critical protein for prostate disease events and support, have upset prostate disease executives in recent years, expanding the existence of a huge number of patients. Be that as it may, made sense of by Dr. Mu, these treatments flop over the long run as prostate growth fosters protection from them.
“We believe that this innovative combination therapy could significantly enhance clinical outcomes in prostate cancer patients and, perhaps, save many lives,”
Study leader Ping Mu, Ph.D., Assistant Professor of Molecular Biology
In the past five years, Dr. Mu and other disease specialists found that one explanation for why these cancers become safe is through a peculiarity considered genealogy pliancy, in which harmful prostate cells return to a prior stage of development and take on another character, turning into an alternate cell type that no longer relies upon the AR. In spite of the fact that ancestry pliancy has been found in other malignant growth types, including cellular breakdown in the lungs, bosom disease, and melanoma, the sub-atomic system behind it has remained generally obscure, obstructing endeavors to foster treatments to keep opposition from developing.
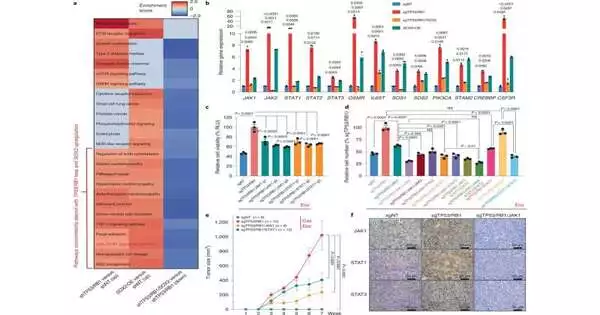
To all the more likely comprehend what drives genealogy versatility and obstruction, Dr. Mu and his associates looked at cells that were safe and delicate to AR-focusing on drugs utilizing different lab models: human prostate malignant growth cell lines filled in petri dishes, three-layered “organoids” made of human and mouse prostate disease cells that imitate the design of normal prostate tumors, and mouse models of prostate disease. Utilizing different scientific techniques, including single-cell RNA sequencing, the analysts looked for key sub-atomic pathways that isolated the safe cells from the touchy ones.
Their hunt uncovered that a specific flagging pathway called Janus kinase-signal transducer and activator of record (JAK-Detail) seemed to drive both genealogy versatility and obstruction. When the scientists utilized a hereditary procedure to independently take out the 11 significant qualities that make up this pathway, they found that qualities known as JAK1 and STAT1 assumed key parts in these peculiarities. Taking out these qualities caused dangerous prostate cells that had moved into new cell types to return to their unique personalities and become delicate to current treatment once more.
Dr. Mu made sense of treating malignant growth cells with drugs that hinder these qualities made a comparable difference, finishing their heredity versatility as well as re-sharpening them to AR-focusing on treatments. When safe prostate malignant growth cells were treated with both JAK1 and STAT1 inhibitors alongside an AR-focusing on drug, these disease cells lost their capacity to partition and get by.
According to Dr. Mu, using a comparative system could offer a better approach to beating obstruction in human prostate disease patients, a methodology he and his colleagues plan to test in a clinical preliminary at the end.
More information: Su Deng et al, Ectopic JAK–STAT activation enables the transition to a stem-like and multilineage state conferring AR-targeted therapy resistance, Nature Cancer (2022). DOI: 10.1038/s43018-022-00431-9
Journal information: Nature Cancer