Scientists at Ume College have found how a specific sort of protein moves for DNA to be duplicated. The revelation could have suggestions for understanding how anti-toxin resistance qualities spread among microbes.
“Concentrating on DNA replication is a decent beginning stage for possibly recognizing focuses for future medication improvement,” says Ignacio Mir-Sanchis, lead scientist in the gathering at Ume College that distributed the review.
All cell creatures should repeat their hereditary material, DNA, to multiply, so one duplicate goes to a girl cell and the other duplicate goes to the next girl cell. The DNA particle can be compared to an extremely lengthy series of dabs, where the dots are the structure blocks or units.
The pearl necklace has two strands that are entwined to frame a winding design, a twofold helix. To copy its hereditary material, the phone should go from one to two DNA particles, a cycle called DNA replication that begins by isolating the two strands of DNA. To isolate the two strands, cells have specific proteins called helicases.
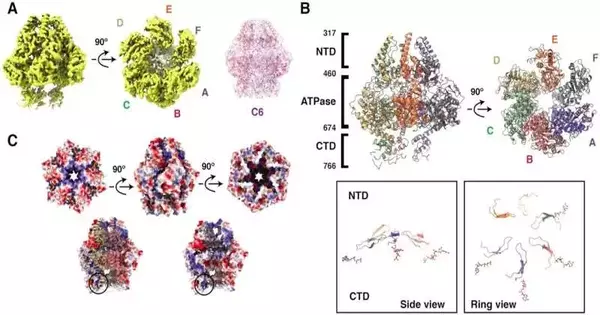
“We discovered that the helicases move various sections, known as domains, in two distinct motions when we examined our images. Two domains rotate and tilt in opposite directions. These movements provide information about how helicases travel on DNA to separate the two strands.”
Cuncun Qiao, a postdoctoral researcher in the team and first author of the paper.
An examination group at the Branch of Clinical Organic Chemistry and Biophysics at Ume College has found how helicases connect and continue on DNA to isolate its strands. The revelation was made conceivable by alleged cryo-electron microscopy, for which Ume has perhaps Sweden’s most developed office. This method permits researchers to take previews of a solitary particle. By joining a great many previews, they can make a film and perceive how the helicases move.
“At the point when we examined our previews, we saw that the helicases move various parts, called areas, through two separate movements. Two areas turn and slant towards one another. “These findings show us how these helicases continue on DNA and separate the two strands,” says Cuncun Qiao, a postdoctoral scientist in the group and the paper’s first author.
Mir-Sanchi’s lab centers around disease science and studies the Staphylococcus aureus bacterium. The analysts are keen on grasping the DNA replication of S. aureus, of infections that taint it (called bacteriophages), and of viral satellites. Viral satellites are infections that parasitize other infections.
S. aureus taints and kills a great many individuals overall and is viewed as a significant danger on the grounds that the bacterium has become impervious to practically all antitoxins. Strangely, the qualities engaged in anti-toxin opposition are at times likewise present in viral satellites, making the work much more medicinally pertinent.
“The discoveries widen how we might interpret how anti-toxin opposition qualities spread, despite the fact that it is important that the developments we have recognized here have likewise been seen in helicases found in eukaryotic infections and, surprisingly, in human cells.” “It’s continuously amazing the way that significant systems are saved from bacteriophages by people,” says Ignacio Mir-Sanchis.
The findings are published in the journal “Nucleic Acids Exploration.”
More information: Cuncun Qiao et al, Staphylococcal self-loading helicases couple the staircase mechanism with inter domain high flexibility, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac625