Juvenile human egg cells skirt a principal metabolic response known to be fundamental for creating energy, as per the discoveries of a concentrate by specialists at the Center for Genomic Regulation (CRG) distributed today in the journal Nature.
By adjusting their metabolic action, the cells try not to make reactive oxygen species, destructive particles that can aggregate, harm DNA and cause cell death. The discoveries make sense of how human egg cells stay lethargic in the ovaries for as long as 50 years without losing their regenerative capacity.
People are brought into the world with all the stockpile of egg cells they have throughout everyday life. Because humans are the longest-living warm-blooded creatures on the planet, their egg cells must maintain perfect conditions while avoiding many years of mileage.We show this issue is settled by skirting a basic metabolic response that is likewise the primary wellspring of harm for the cell. As a drawn-out support technique, it resembles putting batteries in reserve mode. “This addresses a shiny new worldview previously unheard of in creature cells,” says Dr. Aida Rodriguez, postdoctoral analyst at the CRG and the first creator of the review.
“Prior to now, complex I inhibitors were suggested as a cancer treatment. These inhibitors might be able to target malignant cells while preserving oocytes if further research demonstrates their potential.”
Dr. Elvan Böke, senior author of the study and Group Leader in the Cell & Developmental Biology program
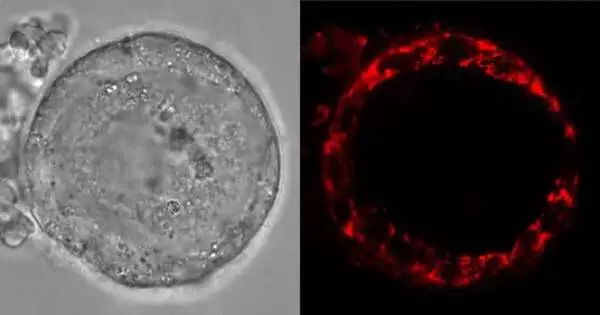
Human eggs are first shaped for quite a while during the fetal turn of events, going through various phases of development. During the beginning phases of this cycle, youthful egg cells known as oocytes are placed into cell capture, staying lethargic for as long as 50 years in the ovaries. Like any remaining eukaryotic cells, oocytes have mitochondria (the batteries of the phone), which they use to create energy for their necessities during this time of lethargy.
Utilizing a blend of live imaging, proteomic, and natural chemistry strategies, the creators of the investigation discovered that mitochondria in both human and Xenopus oocytes utilize elective metabolic pathways to produce energy, previously unheard of in other creature cell types.
A complicated protein and compound known as perplexing I is the standard “guardian” that starts the responses expected to create energy in mitochondria. This protein is basic, working in the cells that comprise living organic entities, from yeast to blue whales. In any case, the analysts observed that complicated I is essentially missing in oocytes. The main other sort of cell known to get by with drained complex I levels are the cells that make up the parasitic plant mistletoe.
As per the creators of the review, the examination makes sense of why a few ladies with mitochondrial conditions connected to complex I, like Leber’s Hereditary Optic Neuropathy, don’t encounter diminished richness contrasted with ladies with conditions influencing other mitochondrial respiratory edifices.
The discoveries could likewise prompt new procedures that assist with protecting the ovarian stores of patients going through malignant growth treatment. “Complex I inhibitors have recently been proposed as a disease treatment. Assuming that these inhibitors show guarantee in later examinations, they might actually target dangerous cells while saving oocytes, “makes sense,” says Dr. Elvan Böke, senior creator of the review and Group Leader in the Cell and Developmental Biology program at the CRG.
Oocytes are immensely unique to different kinds of cells since they need to offset life span with capability. The specialists intend to proceed with this line of examination and uncover the energy source oocytes use during their long lethargy without even a trace of complexity, with one of the points being to figure out the impact of nourishment on female ripeness.
One out of four instances of female fruitlessness are unexplained, highlighting a tremendous hole of information in how we might interpret female proliferation. Our desire is to find the methodologies (like the absence of mind boggling I) oocytes utilize to remain smart for a long time to figure out why these systems at last come up short with old age,” closes Dr. Böke.
More information: Elvan Böke, Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I, Nature (2022). DOI: 10.1038/s41586-022-04979-5. www.nature.com/articles/s41586-022-04979-5