Monitoring potential adverse effects of new Alzheimer’s medicines is an essential part of patient care and drug research. A new article in RadioGraphics, a journal of the Radiological Society of North America (RSNA), investigates the use of monoclonal antibody therapies for the treatment of Alzheimer’s disease and warns physicians about a potential side effect: amyloid-related imaging abnormalities (ARIA).
Alzheimer’s disease is a gradual, irreversible brain illness that causes memory and cognitive function to deteriorate over time. It is the most common type of dementia in the world. While prior treatments focused on symptoms of Alzheimer’s disease, recent approvals of monoclonal antibodies have opened the door to targeting the underlying illness itself.
The primary pathologic hallmark of Alzheimer’s disease is the accumulation of toxic amyloid-B. Disease-modifying medications, like as monoclonal antibodies, function by ridding the brain of toxic amyloid-B protein. The United States Food and Drug Administration (FDA) granted accelerated approval for aducanumab (Aduhelm) as a therapy for Alzheimer’s disease in June 2021. The FDA concluded that there is significant evidence that aducanumab decreases amyloid-B plaques in the brain and that this reduction is likely to benefit patients.
It is essential for the radiologist to recognize and monitor ARIA. As the use of monoclonal antibodies becomes more widespread, close collaboration between neurologists and radiologists is needed before and during therapy to plan for image monitoring per established guidelines.
Amit K. Agarwal
“FDA-approved drugs such as aducanumab, as well as upcoming newer-generation drugs, have provided an exciting new therapy focused on reducing the amyloid plaque burden in Alzheimer’s disease,” said Amit K. Agarwal, M.B.B.S., M.D., lead author of the article and neuroradiologist at Mayo Clinic in Jacksonville, Florida.
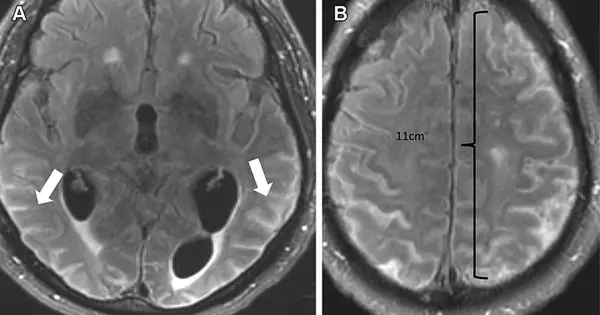
Although this innovative new medication has shown promise in Alzheimer’s sufferers, it is not without risks. Because of the increased use of monoclonal antibodies, amyloid-related imaging abnormalities (ARIA) were discovered. The abnormalities are further categorized into two types: ARIA-E, which represents edema (swelling) and/or effusion, and ARIA-H, which represents hemorrhage. ARIA is hypothesized to be caused by increased vascular permeability after an inflammatory reaction, which allows blood products and fluid to escape into surrounding tissues.
ARIA patients occasionally develop headaches, although they are usually asymptomatic and can only be diagnosed with an MRI.
“It is essential for the radiologist to recognize and monitor ARIA,” Dr. Agarwal said. “As the use of monoclonal antibodies becomes more widespread, close collaboration between neurologists and radiologists is needed before and during therapy to plan for image monitoring per established guidelines.”
The most prevalent side effect of monoclonal antibody treatment is ARIA-E. ARIA-E was found in 35% of individuals on the approved dose in two phase III trials. These trials also revealed that the majority of ARIA-E cases were clinically asymptomatic, with 98% cleared at follow-up imaging. ARIA-E was most common between three and six months of therapy, with prevalence declining substantially after the first nine months. ARIA-H occurs in approximately 15 to 20% of patients treated with monoclonal antibodies. ARIA-H, unlike ARIA-E, is not transitory and does not resolve with time.
Most patients with asymptomatic ARIA meeting specific radiographic and clinical criteria may continue to receive treatment. The vast majority of patients with ARIA-E can continue therapy either with or without temporary suspension. However, in ARIA-H patients, therapy decisions depend on the severity of ARIA-H and whether it is stabilized. The detection of 10 or more new microhemorrhages requires permanent discontinuation of therapy.
“Immunotherapy is becoming more prevalent in managing dementia, and the recently approved monoclonal antibody therapy offers an exciting new frontier,” said Dr. Agarwal. “Identifying and monitoring ARIA plays a vital role in safety monitoring and management decisions in anti-amyloid monoclonal antibody trials and clinical practice.”
When ARIA is present, a conservative surveillance strategy should be devised using a multidisciplinary approach that involves neurologists and radiologists knowledgeable with the clinical and imaging features of the illness, according to Dr. Agarwal.