Methanol, created from carbon dioxide in the air, can be utilized to make carbon-neutral fuel. Yet, to do this, the system by which methanol is transformed into fluid hydrocarbons should be better understood so the reactant cycle can be advanced. Analysts from ETH Zürich and the Paul Scherrer Institute have now gained unique insight into this complex system by employing cutting-edge logical methods.
As we battle to shuffle the effect of outflows with our craving to keep up with our energy-hungry way of life, involving carbon dioxide in the air to make new fills is an energizing, carbon-unbiased alternative. One method for doing this is to make methanol from carbon dioxide in the air by utilizing a cycle called hydrogenation. This methanol can then be changed into hydrocarbons. Although these are then singed, delivering carbon dioxide, this is adjusted via carbon dioxide caught to make the fuel.
To completely foster this feasible fuel, a more profound understanding of the system by which methanol—in a response catalyzed by zeolites, strong materials with novel permeable models—is transformed into long-chain hydrocarbons is vital. In view of this, at the edge of NCCR Catalysis, a Swiss National Center of Competence in Research, scientists from ETH Zürich united with specialists from the Paul Scherrer Institut PSI to uncover the subtleties of this response system, the discoveries of which are published in the journal Nature Catalysis.
“Information is critical for the development of more selective and stable catalysts. Prior to our study, despite many efforts, important mechanistic components of the complex transformation of methanol into hydrocarbons were not well known.”
Javier Pérez-Ramírez, Professor of Catalysis Engineering at ETH Zürich
“Data is vital to growing more specific and stable impetuses,” makes sense to Javier Pérez-Ramrez, Professor of Catalysis Engineering at ETH Zürich and head of NCCR Catalysis, who co-authored the review. “Before our review, in spite of numerous endeavors, key robotic parts of the perplexing change of methanol into hydrocarbons were not surely known.”
The analysts were keen on contrasting the methanol with hydrocarbon process with another cycle: that of transforming methyl chloride into hydrocarbons. Petroleum processing plants regularly consume huge amounts of undesirable methane-rich flammable gas. This dirtying and inefficient action brings about the common flares related to petroleum processing plants. “Transforming methyl chloride into hydrocarbons is a sort of scaffold innovation,” which makes sense for Pérez-Ramrez. “Obviously, we might want to create some distance from petroleum products, but in the meantime, this would be a method for trying not to squander the huge stores of important methane.”
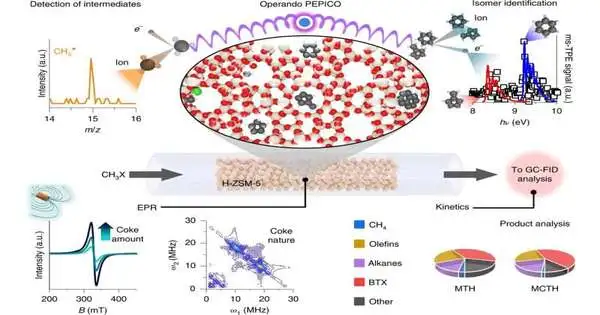
Brief gas stage atoms recount the story.
The key to understanding complex response systems, for example, is to identify the various species included, including the middle items. Customary methods gaze straight toward the outer layer of the impetus to grasp the response, yet a significant piece of the story is told by gas stage atoms, which fall off the impetus.
“These particles are frequently profoundly receptive and brief, decaying in a couple of milliseconds.” This makes recognizing them a genuine test, as customary gas stage logical strategies are just excessively sluggish, “makes sense to Patrick Hemberger, researcher at the vacuum bright (VUV) beamline of the Swiss Light Source SLS, whose modern insightful methods would empower the scientists to concentrate on the response as it worked out.
At the VUV beamline, Photoion Photoelectron Coincidence (PEPICO) spectroscopy has as of late been laid out as a strong logical device in synergist responses. It joins two unique logical methods, photoelectron spectroscopy and mass spectrometry, to give definite data on the gas stage response intermediates, in any event, empowering separation between isomers.
“Because we continuously collect two distinct types of data, we can quickly recognize these brief species even in a blend containing 100 response intermediates and items.”This gives us an uncommon knowledge that is just absurd with regular strategies, “Hemberger says.”
Response pathways are uncovered.
The spectroscopy empowered the analysts to uncover how the carbon bond structure and the hydrocarbon chain develop by recognizing various middle items. For the two cycles—methanol to hydrocarbon and methyl chloride to hydrocarbon—the analysts saw that different response intermediates were happening. From this, they could recognize two particular response pathways: one driven by methyl extremists, present in the two responses, and one more determined by oxygenated species, alleged ketenes, which happened exclusively in the methanol to hydrocarbon response.
The scientists were likewise ready to grasp an intriguing element of the responses: following a few days, the impetus was deactivated and the response halted. This was a result of the development of an undesirable side-effect — coke, which is produced using huge fragrant hydrocarbons stored during the response.
With the assistance of another spectroscopic method, electron paramagnetic reverberation spectroscopy, the analysts saw that the methyl chloride to hydrocarbon creation was considerably more inclined to coke development than creation from methanol. Equipped with information on the response pathways, the justification for this distinction was clear: The methanol to hydrocarbon course continues along two response pathways, while the methyl chloride to hydrocarbon course can take the more receptive methyl extremist course, which is more inclined to shaping coke,” makes sense of Gunnar Jeschke, whose group at ETH Zürich played out the electron paramagnetic reverberation spectroscopy studies.
Understanding the system to advance the cycle
The knowledge acquired by this study is fundamental for the future improvement of fluid filling in a feasible way. This could incorporate tracking down ways of improving the oxygen-driven pathway, hence stifling the arrangement of coke.
With this information, “We currently have a more profound understanding of the response system of methanol to hydrocarbons or methyl chloride to hydrocarbons, and with this information we can upgrade the modern cycle in a designated manner to make it more effective,” adds Hemberger.
More information: Alessia Cesarini et al, Elucidation of radical- and oxygenate-driven paths in zeolite-catalysed conversion of methanol and methyl chloride to hydrocarbons, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00808-0