Working with small microorganisms, Michigan State University scientists led by Lee Kroos have made a revelation that could have huge ramifications for science.
Scientists have uncovered another way that nature can restrain or turn off significant proteins known as intramembrane proteases, which the group detailed on April 26 in the journal eLife.
Although the specialists made this observation utilizing a model life form, an organism known as Bacillus subtilis, this sort of protein is exceptionally well preserved, which is the way transformative scientists say, “it’s all over the place.”
“Our research provides the first example of regulating an intramembrane protease with natural inhibitor proteins. It provides us some thoughts for how we might be able to use and duplicate that.”
said Kroos, a professor in the Departments of Biochemistry and Molecular Biology and Microbiology and Molecular Genetics at the College of Natural Sciences.
These kinds of proteases are found in living beings that span the realms of life, from single-celled microscopic organisms to individuals. Truth be told, the first intramembrane protease was found quite a while ago in 1997, and maybe the most popular individual from this family, named gamma-secretase, is embroiled in Alzheimer’s illness.
“Our paper shows the principal instance of directing an intramembrane protease with normal inhibitor proteins,” said Kroos, a teacher in the College of Natural Science’s Department of Biochemistry and Molecular Biology and Department of Microbiology and Molecular Genetics. “That gives us a few thoughts on how we could possibly utilize and impersonate that.”
Will it let us know how to balance gamma-secretase? No, “Kroos said.” In any case, it could give people a few thoughts on designs they could put on inhibitors to attempt as therapeutics.
Utilizing this data to configure medications to treat Alzheimer’s will require years, Kroos said, but the discoveries could have more immediate effects in battling especially terrible and obstinate bacterial microbes. This includes Bacillus anthracis, the bacterium responsible for Bacillus anthracis contaminations, as well as other microorganisms responsible for lockjaw, botulism, and food contamination.
Many, numerous microscopic organisms have intramembrane proteases that are very firmly connected with the one we examined, said Kroos, who has been an individual of the American Association for the Advancement of Science since around 2014, thanks to a limited extent to his work facilitating how we might interpret science with microorganisms. Assuming we sort those out, we could probably figure out some way to make microorganisms less sensitive to pressure and more treatable with anti-toxins.
A superior understanding of these proteases could likewise assist in creating applications in different regions, including farming and ecological security, eLife noted in an overview highlighting the scientists’ work. Past that, it helps illustrate how life works.
Petra Anne Levin, an editorial manager for eLife and a teacher of science at Washington University in St. Louis, said, “This work ought to have a wide impact on how we might interpret the guidelines of this class of proteins across the tree of life.”
Scissors, spores, and Corvettes
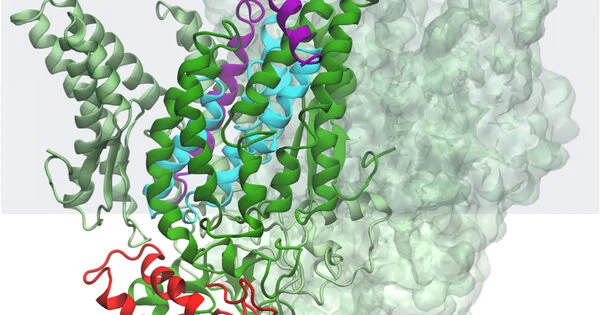
A protease is a compound, a sort of protein machine, that nature uses to slash up different proteins. This is an essential natural cycle that cells use to accomplish an assortment of objectives. An intramembrane protease is a chemical that has its dynamic site—where the compound does the cutting—covered inside a cell layer.
“Now and again you’ll hear them called’scissors in the film,'” Kroos said. “These intramembrane proteases do truly significant things in cells.”
For instance, the protease concentrated by the scientists, for instance, is important for the natural framework that B. subtilis utilizes to make spores when food is scant. Spores are basically torpid cells covered in a protein coating that can survive unforgiving circumstances and then reactivate once things improve (different microscopic organisms, including B. anthracis, likewise structure spores, which is one explanation why these microbes are so industrious).
Since intramembrane proteases go about their responsibilities inside the bounds of a cell layer, it’s been difficult for scientists to decide precisely the way that they work. Adding to the intricacy of the task, the scientists figured their protease may be working in a refined manner that had never been reported.
“At that point, when you take a gander at other related organic entities, you see that this framework has advanced a ton,” Kroos said. “B. subtilis resembles the Corvette.” It has the top of the line hardware. “
Understanding this top of the line hardware required broad hereditary and biochemical testing, which was driven by Sandra Olenic, a doctoral understudy in Kroos’ lab. Olenic procured her Ph.D. in the wake of finishing this undertaking and is presently a postdoctoral researcher at Tufts University.
While Olenic planned and ran tests, she and Kroos understood their outcomes wouldn’t give every one of the responses they looked for. They went to one of Kroos’ long-lasting teammates, Michael Feig, an MSU teacher of organic chemistry and sub-atomic science, to get a PC display and assist with finishing the far-reaching puzzle.
Lim Heo, a postdoctoral examination partner in Feig’s lab, had an ability in a computational procedure that could anticipate protein structures. The procedure has acquired some more extensive consideration as of late on account of Google and other large names in man-made reasoning creating programming bundles that make it more accessible to mainstream researchers.
However, before such apparatuses were accessible, nonetheless, Heo Feig actually had the ability to assist Kroos and Olenic with beginning to sort out a model, making sense of how the intramembrane protease functioned.
“I think this is a truly cool story and a pleasant, coordinated effort that took a ton of difficult work and persistence,” Kroos said. Sandra just had extraordinary ingenuity and commitment. It’s a credit, as well, to Lim and Michael that they did such a pleasant piece of work with the computational displaying. “
Finishing the riddle
Taken by and large, the group’s outcomes infer that this B. subtilis intramembrane protease is kept dormant—that is, not clipping its objective protein or substrate—with assistance from two different proteins. One of these inhibitor proteins works like a clasp, keeping the subsequent protein held up in the scissor catalyst’s dynamic site.
The analysts conjecture that the microscopic organisms can then enact the protease by delivering the clasp, neglecting the shutting protein out and permitting the objective protein in.
“This resembled assembling a 5,000-piece jigsaw puzzle without knowing what it resembled,” Kroos said. Although the riddle isn’t totally settled, the group has an adequate amount of information and results to be certain it has a sensible model of what things look like and work. Yet, the analysts aren’t halting there.
Their subsequent stage is, as of now, in the works, which includes testing the forecasts of the model—for example, where the cinch is appended to both the protease and inhibitor protein—and perceiving how well they match reality. Two undergrad analysts in Kroos’ lab are driving those trials.
Another significant step forward is determining the designs of the proteins involved, which is being done using cutting-edge techniques such as X-beam crystallography and cryogenic electron microscopy.Kroos has been chipping away at that part for about 10 years with colleagues at MSU, yet the proteins are in no rush to surrender their mysteries.
Kroos suspects he might resign before the riddle is completely done, yet that doesn’t seem to irritate him. For one’s purposes, he admitted he could attempt to sneak back and help in the event that his associates would let him. Furthermore, he appears to be eager to see how much of the puzzle he and his group can solve in the few years he has left.
At the point when Kroos began at MSU in 1988, science had close to zero familiarity with intramembrane proteases. However, it wasn’t long after their 1997 disclosure that Kroos concluded he expected to switch his examination concentration to incorporate these proteins.
“That is not something simple to do,” Kroos said, in light of the fact that not everyone — subsidizing offices included — accepted the switch could be made. However, he tracked down help and willing colleagues at MSU.
“Our specialization is truly impressive in film proteins,” he said. “I figured it would be a decent opportunity to blossom where I was planted.”
Any reasonable person would agree that speculation was affirmed.