A novel recycling method for the common plastic found in soda bottles and other packaging has been developed by chemists at CU Boulder. The team’s method uses electricity and clever chemical reactions, and it’s so easy that you can see the plastic break apart right in front of you.
In the journal Chem Catalysis, the researchers presented their novel strategy for chemical recycling.
The global problem of plastic waste is the focus of the study. As per the Natural Security Organization, the US alone created almost 36 million tons of plastic items in 2018. Oana Luca, a co-author of the study, stated that the majority of the waste ends up in landfills.
According to Luca, an assistant professor in the Department of Chemistry, “We pat ourselves on the back when we toss something into the recycling bin, but the majority of that recyclable plastic never winds up being recycled.” We wanted to determine a method for reusing molecular materials, which are the building blocks of plastics.
“We pat ourselves on the back when we toss something into the recycling bin, but the vast majority of recyclable plastic never gets recycled. We wanted to figure out how to recover molecular components, which are the building elements of plastics, so that we could reuse them.”
Luca, assistant professor in the Department of Chemistry.
She and her coworkers got one step closer to doing that with the new research.
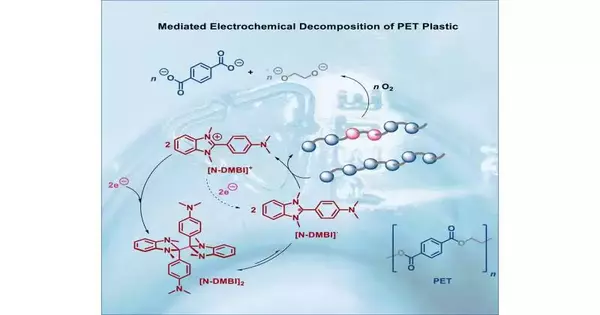
The group focused on polyethylene terephthalate (PET), a type of plastic that is found in water bottles, blister packs, and even some polyester fabrics. The researchers mixed pieces of that plastic with a particular kind of molecule in small lab experiments, then applied a small electric voltage. The PET began to deteriorate quickly.
Before the team’s recycling tool can actually solve the problem of plastic waste around the world, it needs to do a lot more work. According to Phuc Pham, the study’s lead author, watching the waste, which can remain in garbage piles for centuries, vanish in a matter of hours or days was still entertaining.
Doctoral student Pham commented, “It was awesome to actually observe the reaction progress in real time.” As the polymer separates, the solution initially turns a deep pink before becoming clear.
Luca said, “One person’s trash” is a completely new way to look at the possibilities of trash. She said that recycling bins might seem like a good way to solve the problem of plastics all over the world. However, it has been challenging for the majority of municipalities worldwide to collect and sort the small mountain of garbage that people produce each day. The outcome: Short of what 33% of all PET plastic in the U.S. verges on being reused (different sorts of plastic linger much farther behind), Even so, the process of melting or dissolving waste plastic in acid can alter the material’s properties.
In a lab nearby, Phuc Pham applies power to an answer containing ground-up PET plastic. As the plastic begins to dissolve, the solution turns pink. The solution is exposed to oxygen in the final step of the process, which causes it to turn yellow and eventually return to its clear state as the plastic completely breaks down. Credit: College of Colorado at Rock
“You wind up changing the materials precisely,” Luca said. “If you melt a plastic bottle using current recycling methods, you can make, for instance, one of those disposable plastic bags that we now have to pay for at the grocery store.”
She and her group, conversely, need to figure out how to utilize the essential fixings from old plastic containers to make new plastic jugs. It’s like smashing your Lego castle to get the blocks you need to build a new structure.
Another’s treasure The group used electrolysis, or using electricity to separate molecules, to accomplish that feat. For instance, chemists have long known that they can split water molecules into hydrogen and oxygen gas by applying a voltage to beakers containing salts and water.
But PET plastic is much more difficult to separate than water. In the new review, Pham ground up plastic containers and, at that point, blended the powder into an answer. He and his colleagues then added a second ingredient to the solution: a molecule called [N-DMBI]+ salt. Pham explained that this molecule acts as a “reactive mediator” in the presence of electricity and can donate an additional electron to the PET, causing the plastic grains to separate. It is comparable to striking a wooden board with a karate chop in chemistry.
The researchers are still trying to figure out exactly how these reactions happen, but they were able to break down the PET into its fundamental building blocks, which they could then recover and use to make something new.
The researchers said that they could break down about 40 milligrams (a small pinch) of PET over several hours using only tabletop equipment in their lab.
Pham stated, “Although this is a great start, we believe that a great deal of work needs to be done to optimize the process and scale it up so that it can eventually be applied on an industrial scale.”
At the very least, Luca has some big-picture technology ideas.
Luca stated, “I would use these electrochemical methods to break down many different kinds of plastic at once if I were to have my way as a mad scientist.” You could, for instance, travel to enormous garbage patches in the ocean, load all of that garbage into a reactor, and then get a lot of useful molecules back.”
More information: Phuc H. Pham et al, Electricity-driven recycling of ester plastics using one-electron electro-organocatalysis, Chem Catalysis (2023). DOI: 10.1016/j.checat.2023.100675