An exploration effort including scientists from Texas A&M University, the Perelman Institute of Medication at the College of Pennsylvania, and the Kids’ Clinic of Philadelphia (Hack) has utilized human genomics to recognize another hereditary pathway engaged with managing rest from natural product flies to people—aa clever knowledge that could prepare for new medicines for sleep deprivation and other rest-related messes.
Texas A&M geneticist and developmental scholar Alex Keene teamed up with Penn’s Allan Pack and Philip Gehrman and Hack’s Struan Award on the pivotal exploration, which is distributed in Science Advances.
“There have been significant efforts to use human genomic studies to track down rest qualities,” Keene explained.”A few examinations have affected countless people.” However, creature model approval and testing is necessary for grasping capability.We accomplished this here, primarily because each of us brings a unique perspective on the long-term viability of this collaboration.”
“A tremendous amount of work has gone into using human genetic studies to identify sleep-related genes. Numerous research involve tens of thousands of participants. But understanding function requires validation and testing using animal models. Our is what we’ve done here, largely because each of us has a unique set of skills that has made this partnership ultimately successful.”
Biologist Alex Keene
Keene says the most thrilling thing about the cooperation is that they fostered a pipeline beginning not with a model creature but rather with genuine human genomic information.
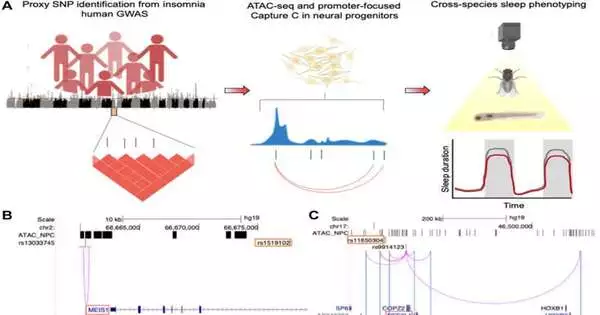
“There is a wealth of human vast affiliation studies (GWAS) that recognize hereditary variations related to rest in people,” Keene said. “In any case, approving them has been a huge test.” Our group utilized a genomics approach called variation-to-quality planning to foresee the qualities influenced by each hereditary variation. Then we screened the impact of these qualities on organic product flies.
“Our examinations found that changes in the quality of Pig-Q, which is expected for the biosynthesis of a modifier of protein capability, led to expanded rest.” We then tested this in a vertebrate model, the zebrafish, and found a similar effect.”As a result, Pig-Q is related to the rest guideline in humans, flies, and zebrafish.”
Keene says the group’s next stage is to concentrate on the job of a typical protein change, GPI-anchor biosynthesis, on the rest guideline. Furthermore, he mentions that the human-to-organic product travels to the group’s zebrafish pipeline, which will enable them to practically survey rest qualities as well as different attributes normally focused on utilizing human GWAS, for example, neurodegeneration, maturing, and memory.
“Understanding how qualities direct rest and the job of this pathway in the rest guideline can assist with opening future discoveries on endlessly rest issues, like sleep deprivation,” said Gehrman, an academic partner in clinical brain science in psychiatry at Penn and a clinical clinician with the Penn Chronobiology and Rest Foundation. “Pushing ahead, we will proceed to utilize and concentrate on this framework to recognize more qualities managing rest, which could point toward new medicines for rest issues.”
Keene’s examination inside his Middle for Natural Clocks Exploration-partnered lab lies at the convergence of development and neuroscience, with an essential spotlight on understanding the brain systems and transformative underpinnings of rest, memory arrangement, and other social capabilities in fly and fish models. He focuses on natural product flies (Drosophila melanogaster) and Mexican cavefish that have lost both their vision and their ability to lay down completely intent on recognizing the hereditary premise of social decisions that factor into human illness, such as weight, diabetes, and coronary illness.
More information: Justin Palermo et al, Variant-to-gene-mapping followed by cross-species genetic screening identifies GPI-anchor biosynthesis as novel regulator of sleep, Science Advances (2023). DOI: 10.1126/sciadv.abq0844. www.science.org/doi/10.1126/sciadv.abq0844