Researchers all over the planet are exploring the way that enemy of disease medications can most efficiently arrive at the growth targets they target. One chance is to involve altered microorganisms as “ships” to bring the medications through the circulatory system to the cancers. Scientists at ETH Zurich have now prevailed with regards to controlling specific microbes so they can actually cross the vein wall and penetrate growth tissue.
Driven by Simone Schürle, Teacher of Responsive Biomedical Frameworks, the ETH Zurich analysts decided to work with microbes that are normally attractive because of the iron oxide particles they contain. These microorganisms of the variety Magnetospirillum respond to attractive fields and can be constrained by magnets from outside the body.
Taking advantage of impermanent holes
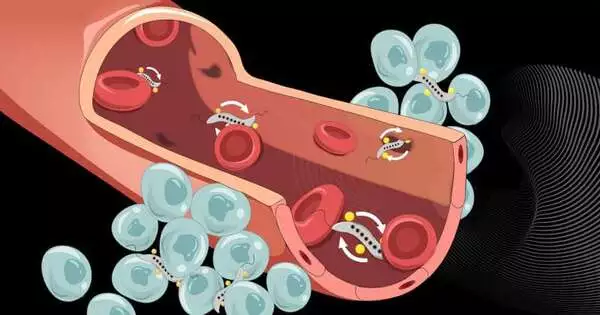
In cell societies and in mice, Schürle and her group have now shown that a pivoting attractive field applied at the growth works on the microbes’ capacity to cross the vascular wall close to the harmful development. At the vascular wall, the turning attractive field impels the microorganisms forward in a round movement.
An itemized look is important to all those more likely to comprehend how the system to cross the vessel wall works. The vein wall comprises a layer of cells and fills in as an obstruction between the circulatory system and the cancer tissue, which is pervaded by many little veins. Restricted spaces between these cells permit specific particles from them to go through the vessel wall. How huge these intercellular spaces are is controlled by the cells of the vessel wall, and they can turn out to be briefly adequate to permit even microscopic organisms to pass through the vessel wall.
“Solid impetus” and “high likelihood”
The ETH Zurich scientists were able to demonstrate that impelling microscopic organisms using a turning attractive field is successful for three reasons using research and programmatic experiences.In the first place, driving by means of a turning attractive field is multiple times more impressive than impetus through a static attractive field. The last option only sets the bearing, and the microbes need to move under their own power.
“We also leverage the bacteria’s natural and autonomous movement. Once the bacteria have slipped through the blood vessel wall and are inside the tumor, they can travel deep into its interior on their own.”
Simone Schürle, Professor of Responsive Biomedical Systems,
The second and most basic explanation is that microorganisms driven by the pivoting attractive field are continually moving, going along the vascular wall. This makes them bound to experience the holes that momentarily open between vessel wall cells, contrasting with other drive types, in which the microorganisms’ movement is less explorative. What’s more, third, not at all like different techniques, the microscopic organisms needn’t bother with following through on the picture. When the attractive field is over the cancer, it does not need to be straightened out.
‘Freight’ gathers in growth tissue
“We utilize the microscopic organisms’ normal and independent movement too,” Schürle makes sense of. “When the microorganisms have gone through the vein wall and are in the growth, they can autonomously move profoundly into its inside.” Thus, the researchers utilize the drive by means of the outer attractive field for only one hour—long enough for the microscopic organisms to go through the vascular wall and arrive at the growth proficiently.
Such microbes could transmit hostile to malignant growth drugs from here on out. In their cell culture studies, the ETH Zurich scientists recreated this application by appending liposomes (nanospheres of fat-like substances) to the microbes. They labeled these liposomes with a fluorescent color, which permitted them to exhibit in the Petri dish that the microscopic organisms had to be sure conveyed their “freight” inside the malignant tissue, where it collected. In a future clinical application, the liposomes would be loaded with medication.
Bacterial disease treatment
Involving microscopic organisms as carriers for drugs is one of two different ways that microorganisms can help in the battle against disease. The other methodology is over 100 years old and right now experiencing a restoration: utilizing the normal inclination of specific types of microscopic organisms to harm cancer cells. This might include a few systems. Anyway, it is realized that the microbes invigorate specific cells of the safe framework, which then, at that point, take out the growth.
Various examination projects are, as of now, researching the viability of utilizing E. coli microbes against cancers. Today, it is feasible to change microbes utilizing manufactured science to enhance their helpful impact, lessen secondary effects, and make them more secure.
Making non-attractive microscopic organisms attractive
However, to involve the innate properties of microbes in disease treatment, the subject of how these microscopic organisms can achieve productive growth remains. While it is feasible to infuse the microbes straightforwardly into cancers close to the outer layer of the body, this isn’t workable for growths somewhere inside the body. That is where Teacher Schürle’s microrobotic control comes in. “We accept we can utilize our designing way to deal with increasing the viability of bacterial malignant growth treatment,” she says.
E. coli utilized in the malignant growth studies isn’t attractive and, in this manner, can’t be moved and constrained by an attractive field. As a general rule, attractive responsiveness is an extremely uncommon peculiarity among microorganisms. Magnetospirillum is one of a handful of genera of microorganisms that have this property.
Schürle thusly needs to make E. coli microscopic organisms attractive too. This could one day make it possible to use an attractive field to control clinically used remedial microorganisms that have no natural attraction.
More information: T. Gwisai et al, Magnetic torque–driven living microrobots for increased tumor infiltration, Science Robotics (2022). DOI: 10.1126/scirobotics.abo0665. www.science.org/doi/10.1126/scirobotics.abo0665
Journal information: Science Robotics