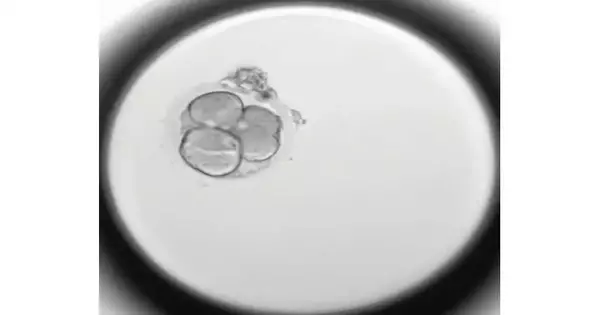
By hereditarily testing almost 1,000 undeveloped organisms, researchers have given the most point-by-point examination of incipient organism destiny following human in vitro preparation.
Almost a portion of the undeveloped organisms contemplated went through formative capture due to hereditary setbacks in the early stages of events—a noteworthy understanding that proposes more IVF children could come to terms with changes in the fruitfulness of the treatment process. The novel mix of information from captured undeveloped organisms likewise reveals new insight into the still, to a great extent, baffling earliest phases of pregnancy through normal means.
“We think this additionally occurs in normal origination, and it’s the reason it requires on average a few or more months to get pregnant,” said creator Rajiv McCoy, an associate teacher of science at Johns Hopkins College.
“It is exceptionally astounding that the majority of these undeveloped organism captures are coming not from blunders in egg development but rather from mistakes occurring in cell divisions after preparation. The way that these mistakes don’t come from the egg suggests that perhaps they could be alleviated by fundamentally impacting how IVF is finished.”
The examination is planned to be distributed through genome medication.
A period-pass clasp of a typical sort of strange cell division where an incipient organism separates straightforwardly from a solitary-celled zygote into three (instead of two) cells The new exploration shows such unusual division is unequivocally connected with chromosome irregularities and incipient organism capture. Credit: Christian Ottolini
Johns Hopkins and London Ladies’ Center scientists in the U.K. contrasted IVF incipient organisms that fizzled with foster inside a couple of long periods of treatment with incipient organisms that made due, searching for hereditary contrasts.
“In order to select the embryo to transfer to the uterus, genetic testing is normally only done on IVF embryos that survive. However, from a biological perspective, evaluating all other embryos is necessary if we want to understand what’s allowing these embryos to survive.”
Author Rajiv McCoy, an assistant professor of biology at Johns Hopkins University.
“Hereditary testing is normally just finished on IVF-undeveloped organisms that get by to choose which incipient organism to move to the uterus,” McCoy said. “Be that as it may, from a scientific viewpoint, if we need to comprehend what’s permitting these undeveloped organisms to get by, then we need to test any remaining incipient organisms as well.”
The discoveries uncover how a few undeveloped organisms begin developing appropriately while the maternal hereditary material is pre-stacked into the egg control cell division, only to waver and slow down when the incipient organism’s qualities dominate.
Human cells ordinarily get 46 chromosomes, 23 from each parent. The group found nonviable, undeveloped organisms began with the 46-chromosome set; however, at that point, they passed down mistaken quantities of chromosomes as cells were isolated.
“It doesn’t exactly make any difference assuming you have extra missing chromosomes in the earliest reference point in light of the fact that the maternal hardware is controlling things,” McCoy said. “At the point when the undeveloped organism’s genome turns on, that is when things turn out badly.”
Human undeveloped organisms experience curiously high rates of chromosome gain and misfortune, known as aneuploidy, in the early stages of events. Researchers have been studying aneuploidy for quite some time by screening undeveloped IVF organisms, and these setbacks are surely known to be the reason for pregnancy misfortune in people. Since aneuploidy is uncommon in numerous different species, McCoy said, the discoveries could assist with making sense of why pregnancy misfortune and premature delivery are so normal in people.
“Aneuploidy is an illustration of a very amazing sort of regular choice that is going on at each age in people,” McCoy said. “It may very well be an element of human multiplication and improvement; however, it has suggestions for IVF. So in the long haul, we trust that we can work on hereditary testing and further develop IVF results.”
The scientists intend to run extra tests on unambiguous cells from captured incipient organisms to follow the chromosomes’ beginnings and see whether unusual cell divisions are connected to maternal or fatherly hereditary qualities. They additionally need to all the more likely comprehend if factors, for example, the compound arrangement in the dish where the undeveloped organisms are developed, could further develop opportunities for endurance.
“We might actually address a ton of these things by seeing more about the hardware that causes undeveloped organism capture,” said co-creator Michael Summers, a senior expert in regenerative medication at the London Ladies’ Center.
“The issue could be that the substance organization of the way of life mediums that are usually utilized won’t permit all undeveloped organisms to develop and that the strange cell divisions are because of weights on the egg and early undeveloped organisms that cause the unusual divisions related to chromosome anomalies.”
More information: Meiotic and mitotic aneuploidies drive arrest of in vitro fertilized human preimplantation embryos, Genome Medicine (2023). DOI: 10.1186/s13073-023-01231-1