Specialists from Tel Aviv College have prevailed with regards to delivering “green” hydrogen utilizing green power. The hydrogen is created without air contamination, with an elevated degree of proficiency, using a biocatalyst. Hydrogen is an essential unrefined substance for both farming and industry, yet 95% of the hydrogen delivered in this present reality is “dark” or “dim—created from coal or flammable gas and transmitting 9–12 tons of carbon dioxide for each huge amount of hydrogen.
The new strategy was created by doctoral understudy Itzhak Grinberg and Dr. Oren Ben-Zvi, under the direction of Prof. Iftach Yacoby of the School of Plant Sciences and Food Security at the Personnel of Life Sciences and Prof. Lihi Adler-Abramovich of the School of Dental Medication and the Middle for Nanoscience and Nanotechnology. The promising examination results were distributed in the diary Carbon Energy, zeroing in on cutting-edge materials and innovation for clean energy and CO2 discharge decrease.
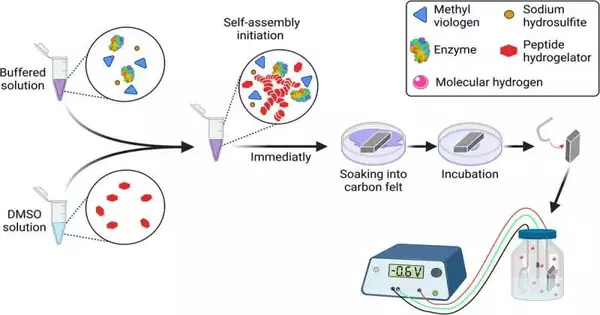
“Hydrogen is extremely uncommon in the air,” makes sense of Itzhak Grinberg, “despite the fact that it is delivered by catalysts in minuscule creatures, which get the energy for this from photosynthesis processes. In the lab, we ‘jolt’ those catalysts; that is to say, a terminal gives the energy rather than the sun. The outcome is an especially productive cycle, with no interest in outrageous circumstances, that can use power from sustainable sources like sunlight-based chargers or wind turbines. In any case, the protein ‘takes off’ from the electric charge, so it should be held in place through synthetic treatment. We tracked down a basic and effective method for connecting the compound to the terminal and used it.”
“The gel’s composition is already understood, but our innovation is using it to generate hydrogen. The gel, which contains hydrogenase, an enzyme for generating hydrogen, was applied to the electrode. In contrast to electrolysis, which requires pure water, the gel maintains the enzyme for a long time even under the electric voltage, allowing for highly efficient hydrogen production in environments that are friendly to the enzyme, such as salt water.
Prof. Iftach Yacoby of the School of Plant Sciences and Food Security at the Faculty of Life Sciences.
The scientists utilized a hydrogel (a water-based gel) to join the chemical to the cathode and had the option to deliver green hydrogen utilizing a biocatalyst with more than 90% proficiency; that is, more than 90% of the electrons brought into the framework were stored in the hydrogen with practically no optional cycles.
Prof. Iftach Yacoby makes sense of that: “The material of the actual gel is known, yet our advancement is to utilize it to deliver hydrogen. We absorbed the cathode in the gel, which contained a compound for delivering hydrogen called hydrogenase. The gel holds the compound for quite a while, considerably under the electric voltage, and makes it conceivable to create hydrogen with extraordinary productivity and in ecological circumstances great for the protein—ffor instance, in salt water, as opposed to electrolysis, which requires refined water.”
Prof. Lihi Adler-Abramovich adds, “Another benefit is that the gel collects itself—yyou put the material in water, and it subsides into nanometric filaments that structure the gel. We demonstrated that these filaments are likewise ready to adhere the chemical to the terminal. We tried the gel with two different compounds, notwithstanding the hydrogenase, and demonstrated that connecting various proteins to the electrode was capable.”
“Today, ‘green’ hydrogen is delivered essentially through electrolysis, which requires valuable and interesting metals, for example, platinum, alongside water refining, which makes the green hydrogen up to multiple times more costly than the dirty ‘dim’ one,” says Dr. Oren Ben-Zvi. “We trust that later on, it will be feasible to utilize our technique economically, to bring down the expenses, and to make the switch towards involving green hydrogen in industry, farming, and as a spotless energy source.”
More information: Itzhak Grinberg et al, Peptide self‐assembly as a strategy for facile immobilization of redox enzymes on carbon electrodes, Carbon Energy (2023). DOI: 10.1002/cey2.411