With regards to conveying medications to the body, a significant test is guaranteeing that they stay in the space they’re treating and proceeding to precisely convey their payload. While significant steps have been made in conveying drugs, checking them is a test that frequently requires obtrusive methodologies like biopsies.
Analysts at NYU Tandon, led by Jin Kim Montclare, Teacher of Substance and Biomolecular Designing, have created proteins that can collect themselves into filaments to be utilized as restorative specialists for possible medicines for different sicknesses.
These biomaterials can embody and convey therapeutics for a large group of illnesses. Yet, while Montclare’s lab has long dealt with creating these materials, there was once a test that was difficult to survive—how to ensure that these proteins kept on conveying their therapeutics to the right area in the body for the essential measure of time.
“This will absolutely accelerate what we can make in terms of biomaterial manufacturing. Traditionally, you make rational adjustments and see if they work, which 90% of the time they don’t. All of them work with this new model, and we can then choose the best of the ones that work. It will change the way we create biomaterials.”
Jin Kim Montclare, Professor of Chemical and Biomolecular Engineering,
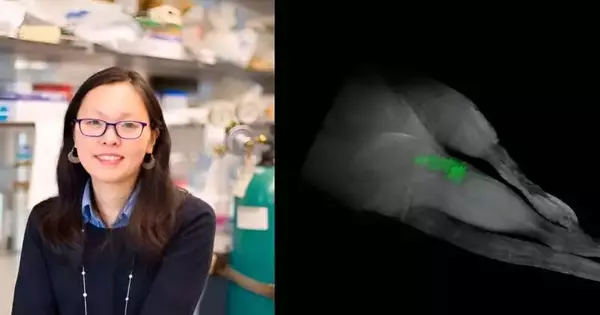
In a new report distributed by the journal ACS Applied Nanomaterials, her lab had the option to make biomaterials that were fluorinated. On account of this fluorination, they can be checked by basic FMRI examinations, permitting clinical experts to guarantee that the medications stay in the treatment regions through painless imaging innovation.
The material is comprised of regular proteins, yet the examination group presented the non-normal aminocorrosive trifluoroleucine. Since fluorine is uncommon in the body, it permits the biomaterials to illuminate like an occasion show when the body is placed into a 19FMRI sweep.
“As a theranostic specialist, it can not just convey a helpful solution for malignant growth or joint illness, for instance; however, we can now see that it’s still set up in the body and delivering the prescription where it should,” Montclare says. “It eliminates the requirement so that obtrusive medical procedures or biopsies all together might be able to see what’s happening.”
Montclare’s lab carries out momentous analysis in designing proteins to impersonate nature and, now and again, work better compared to nature. She attempts to modify fake proteins determined to target human issues, drug conveyance, and tissue recovery, as well as make nanomaterials for gadgets. Using science and hereditary design, she has made commitments to sicknesses ranging from coronavirus to osteoarthritis to some more.
This advanced purpose is the very amino acids and proteins that portray a lot of Montclare’s exploration. Since they are made of natural materials, when these biomaterials have finished their task and conveyed therapeutics, the body can separate them with next to no sort of antagonistic impact.
This isolates it from different medicines that utilize non-natural materials that could cause an extreme resistance reaction or different responses. In combination with the fluorination procedure, these materials could provide a treatment for restricted illnesses that is undeniably less obtrusive than current medicines and is far more straightforward and less problematic to screen.
Montclare worked intimately with NYU Institute of Medication personnel on this review, including co-relating creator Youssef Z. Wadghiri in the Radiology branch as well as Richard Bonneau at the Flatiron Establishment.
Montclare’s group showed their exploration in mouse models, yet she is as of now hoping to probe mice with explicit problems to demonstrate the protein’s abilities to treat illnesses.
The proteins that Montclare’s group utilized are just a subset of what she and her lab are dealing with. In one more paper distributed in Biomacromolecules, her lab had the option to utilize a computational plan to make proteins that could frame hydrogels because of a program composed by her Ph.D. understudy, Dustin Britton.
These hydrogels have different change temperatures—the temperature at which the gels can remain gelled without dissolving or becoming unsound. Beforehand, the maximum furthest reaches of gelation were around 17°C. For biomedical applications, this was less than ideal, as it would liquefy as it moved toward the human internal heat level. Using his computationally planned proteins, Britton had the option to move this limit up to 33.6° Celsius.
In light of this new security, the proteins that Britton and Montclare planned could be utilized for skin medicines, including recuperating wounds. Also, notwithstanding the expanded intensity resistance, the new protein can gel a lot quicker than past variants, making it undeniably more productive and more helpful for clinical applications.
While moving the temperature, Britton was likewise ready to plan a protein that is additionally fluorescent, implying that it has a similar potential for representation as the fluorinated proteins in their other review. That permits specialists to screen for its presence in injuries and guarantee it’s conveying its helpful payload. What’s more, the gel has similar advantages to the lab’s proteins implied for inside use, in that it will actually want to debase and disseminate in the body with few to no evil impacts.
Britton’s PC model is accomplishing more than just planning this particular protein. As indicated by Monclare, the field of protein-designed biomaterials has for some time been overwhelmed by experimentation—testing speculative plans to check whether they’ll be steady. Be that as it may, Britton’s model had the option to make reliably effective gels, producing groupings with a very high achievement rate and making new proteins with new properties for expected helpful purposes.
“For biomaterial production, this will totally speed up what we’re ready to make,” says Montclare. “How it’s customarily finished, you roll out normal improvements and check whether it works, and 90 percent of the time, it doesn’t. With this new model, every one of them works, and we can then pick from the best of the ones that work. It will change the manner in which we make biomaterials.”
In Monclare’s lab, this has meaningfully impacted the manner in which they’ll make new proteins and materials going forward—pressing forward is the only real option to the normal emphasis practice that had such a high disappointment rate. Also, it will unquestionably speed up the development of progressive biomaterials that will, before long, be mending probably the most serious ailments around the world.
More information: Dustin Britton et al. Protein-Engineered Fibers for Drug Encapsulation Traceable via 19F Magnetic Resonance, ACS Applied Nano Materials (2023). DOI: 10.1021/acsanm.3c04357
Dustin Britton et al, Computational Prediction of Coiled-Coil Protein Gelation Dynamics and Structure, Biomacromolecules (2023). DOI: 10.1021/acs.biomac.3c00968