Compound responses frequently result in disorganized combinations of various items.As a result, scientists expend a great deal of effort persuading their responses to be more specific in order to produce specific objective particles.Presently, a global group of scientists has accomplished that sort of selectivity by conveying voltage heartbeats to a solitary particle through a staggeringly sharp tip.
“Controlling the pathway of a compound response, contingent upon the voltage beats utilized, is uncommon and extremely charming to scientists,” says KAUST’s Shadi Fatayer.
The group utilized an instrument that combines checking burrowing microscopy (STM) and nuclear power microscopy (AFM). The two methods can outline the places of iotas inside individual particles by utilizing a tip that might be only a couple of molecules wide. Yet, the voltage can likewise be utilized to break bonds inside a particle, possibly permitting new bonds to be formed.
“Tip-controlled responses have been recently performed, yet there was zero power over the end result,” Fatayer says. “The selectivity is the vital component here—contingent upon the extremity and worth of the voltage beats, we can shape and break different inner bonds freely.”
“Previously, tip-controlled reactions were carried out, but there was no control over the final result. The crucial feature here is selectivity—we may establish and break distinct internal links at whim based on the polarity and value of the voltage pulses.”
Shadi Fatayer
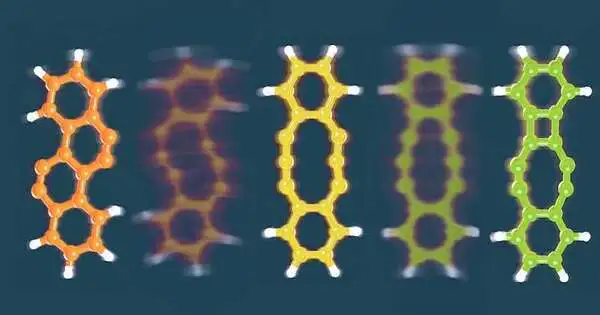
The scientists utilized this way to deal with studying tetrachlorotetracene, a particle that contains four chlorine iotas joined to a line of four hexagonal rings of carbon molecules. Applying a voltage of around 3.5V eliminated two chlorine iotas and incited the particle to revamp. Expanding the voltage eliminated the excess chlorine iotas, setting off additional revisions that framed three unique items.
The main item has four hexagonal rings organized in a crisscross pattern; the second has a 10-membered ring flanked by two six-membered rings; and the third contains a four-membered ring, an eight-membered ring, and two six-membered rings.
Little voltage heartbeats could be utilized to interconvert these items. By tweaking the voltage, the analysts had some control over which bonds were broken and which revamped items shaped.
Combining their outcomes with hypothetical estimations, the analysts showed that the strategy’s selectivity relies upon the state of energy the atoms take on when they convey different electrical charges, known as their oxidation state. Fatayer says that since the underlying oxidation condition of a particle can be constrained by an electric field, this approach could assist scientists with planning new compound responses and items. This examination was included on the intro page of Science.
His group is currently creating ways of adding or eliminating single electrons to individual particles, and applying voltage heartbeats to explicit pieces of an atom to control which compound response happens.
More information: Florian Albrecht et al, Selectivity in single-molecule reactions by tip-induced redox chemistry, Science (2022). DOI: 10.1126/science.abo6471
Journal information: Science