College of Focal Florida material sciences engineers Melanie Coathup and Sudipta Seal have planned a cerium oxide nanoparticle—a fake protein—that shields bones against harm from radiation. The nanoparticle has likewise shown the capacity to work on bone recovery, reduce loss of platelets and assist with killing disease cells.
Their review, a collaboration with Oakland College, North Carolina A&T College, the College of Sheffield, and the College of Huddersfield in the U.K., was distributed in Bioactive Materials.
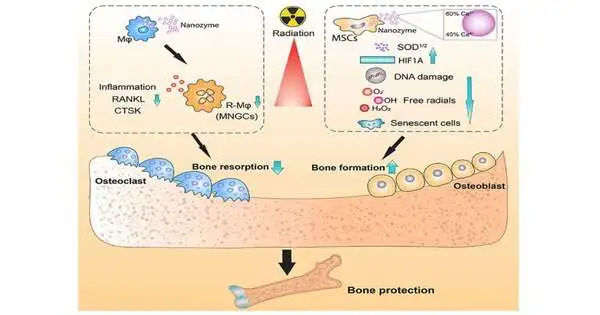
Approximately half of all disease patients receive radiation therapy, which uses electrically charged particles to kill malignant growth cells.Around 40% of patients are restored with this treatment. In any case, bone harm is a secondary effect, affecting around 75% of patients getting radiation.
“There is currently no effective medicine or therapy available to protect healthy tissue from radiation harm. This is an issue not only for cancer patients undergoing treatment, but also for astronauts and future deep space travel.”
Material sciences engineers Melanie Coathup
“Due to its high calcium content, bone ingests 30-40% more radiation than different tissues, thus it is a typical site of injury,” says Coathup, chief of UCF’s Biionix staff group. “Radiation makes the bone weak and handily breaks. Also, because of the harm brought about by radiation, many individuals are then unfit to fix their broken bones. In certain individuals, this prompts a removal to determine the confusion.
While radiotherapy radiation is clearly directed at the tumor, surrounding solid tissue is also damaged, which can result in a variety of additional medical issues for patients.
“Right now, there is no genuine medication or treatment to shield solid tissue from the harm brought about by radiation,” Coathup says. “This isn’t just an issue for disease patients who go through radiotherapy, but in addition, it presents issues for space travelers and future profound space exploration.”
The body’s normal guard against radiation is a gathering of proteins called cell reinforcements—yet this safeguard framework gets handily wrecked by radiation and, all alone, can’t shield the body from harm. Seal, a main nanotechnologist, planned the cerium oxide nanoparticle—or nanoceria—that copies the action of these cell reinforcements and has a more grounded guard system in safeguarding cells against DNA harm.
“The nanoceria works with an explicitly planned regenerative grid structure liable for obliterating unsafe receptive oxygen species, a result of radiation therapy,” Seal says.
Working with postdoctoral analyst Fei Wei, Coathup tried the nanozyme in live models getting radiation treatment.
“Our review showed that presenting rodents to radiation at comparable levels to those given to disease patients prompted frail and harmed bones,” Coathup says. “Nonetheless, when we treated the creatures with the nanozyme, previously and during three dosages of radiation north of three days, we observed that the bone was not harmed, and had a strength like sound bone.”
The concentrate likewise showed that the nanozyme therapy helped kill disease cells, perhaps because of an expansion in acridity, and safeguarded against the deficiency of white and red platelets that normally happens in malignant growth patients. A low white and red platelet count implies the patient is more helpless to disease, less ready to battle malignant growth and is more exhausted. Another intriguing find is that the nanoparticle likewise improved sound cells’ capacity to create more cancer prevention agents, decreased irritation (which additionally prompts bone misfortune), and advanced bone development.
Future exploration will try to decide the proper dose and organization of the nanozyme and further investigate how the nanozyme assists with killing disease cells. The analysts will likewise concentrate their examinations with regard to bosom disease, as ladies are more vulnerable to bone damage than men.
“Malignant growth patients are now battling more than one illness,” Coathup says. “They shouldn’t need to be stressed over bone cracks and tissue harm. So we’re trusting this leading edge will assist survivors with returning to carrying on with a typical and solid life. “
More information: Fei Wei et al, A novel approach for the prevention of ionizing radiation-induced bone loss using a designer multifunctional cerium oxide nanozyme, Bioactive Materials (2022). DOI: 10.1016/j.bioactmat.2022.09.011