A newfound procedure, revealed in the diary Nanoscale, offers a minimal-expense method for upgrading the viability of existing medications.
“Assuming you take sand and heat it to 500 degrees Celsius, nothing changes,” said Bradley Smith, the Emil T. Hofman Teacher of Science at the College of Notre Dame.
So Smith, who is likewise the overseer of Notre Dame’s Coordinated Imaging Office, was perplexed when Canjia Zhai and Cassandra Shaffer, two doctoral understudies in the Branch of Science and Organic Chemistry who were working in his lab, found they had changed the design of particles of silica—tthe primary part of sand—aat 80 degrees Celsius, a temperature like that of some espresso.
The revelation occurred unintentionally. The particles were infinitesimally a thousandth the measurement of a human hair. In any case, similar to their bigger partners stamped “silica gel” in bundles joined to new pieces of clothing, these particles were permeable and could hold a compound. For this situation, that substance was a blue color used to identify growths in mice.
“These microorganisms have systems that allow them to transform sand into shells. They clearly do it at a low temperature by employing organic molecules. We may have found some of the chemistry involved in that process.”
Bradley Smith, the Emil T. Hofman Professor of Science at the University of Notre Dame.
The new color, which had been created in Smith’s lab, was consuming a large chunk of the day to enter the thin pores in the particles. To make the particles move faster, Shaffer and Zhai warmed the mixture to a state of simple bubbling and left it for a short time.When they returned the following day, they could see that the particles had turned blue.
To affirm that the color had been completely injected, Shaffer and Zhai enlisted the assistance of Tatyana Orlova and Maksym Zhukovskyi, microscopy specialists at the Notre Dame Incorporated Imaging Office.
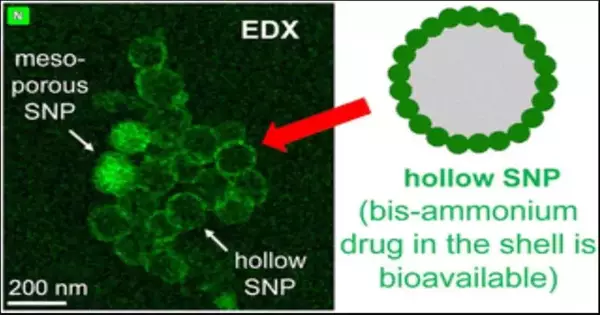
Orlova and Zhukovskyi used high-resolution electron microscopy to show that the silica particles’ shape had changed, not just their color.The first particles were singular circles delicately speckled with pores like the skin of an orange. The new designs were circular and made of lighter-colored filled globules.They also had numerous small openings that revealed an empty center inside.The general unit looked like an empty raspberry.
After the shock of the underlying revelation, there were various viable inquiries. What different synthetic substances might the analysts at any point stack into comparable raspberry-molded particles? What’s more, above all, could those synthetics stay dynamic even after their encompassing designs had changed shape?
Jordan Chasteen, an individual doctoral understudy, took up these inquiries, rehashing the cycle with a disease drug.After a series of tests, he confirmed that the malignant growth drug stacked into the particles was still active and capable of killing disease cells.
This revelation offers another device for making existing medications more powerful, Smith said.
“What we have now is a best approach through the entire index of amine-containing drugs, and by following the straightforward advances we have found, we can make new forms of existing medications that could be more viable or have fewer undesirable side effects,” he said.
Smith and his understudies have found that unobtrusive changes in the stacking strategy permit them to fluctuate the thickness of the particles, offering an entire host of new choices to tweak the particles to deliver drugs at various rates. The new molecule’s one-of-a-kind structure may also enable scientists to stack it with more than one fixing—for example, a medication in the outer layer and a color inside the “raspberry”—to improve scientists’ capacity to notice how medications discharge.
Furthermore, Smith claims that the new molecule sheds light on a previously unknown natural phenomenon known as biomineralization.
“We have found that amine-containing drugs have specific substances that accelerate the corruption and transforming process in silica, and we imagine that it happens in nature,” he said. Smith specifies as an illustration diatoms, a sort of microscopic fish, and their fragile glass-like shells framed from silica.
“These microorganisms have systems that permit them to take sand and rebuild it into their shells,” he says. “They also obviously do it at a moderately low temperature using natural particles.””What we have found is possibly a portion of the science behind that cycle.”
As Smith and his lab keep improving, they are acquiring motivation both from nature and from revelations in the lab. “The expansive example here,” he says, “is that we can find in the lab how regular cycles work, and afterward we can utilize that information and copy those cycles to plan something totally new.”
More information: Cassandra C. Shaffer et al, Silica nanoparticle remodeling under mild conditions: versatile one step conversion of mesoporous to hollow nanoparticles with simultaneous payload loading, Nanoscale (2022). DOI: 10.1039/D2NR05528G
Journal information: Nanoscale