Johns Hopkins medication specialists have patched up an enemy of malignant growth medication to more readily target disease cells and leave sound tissues safe. Researchers have dubbed this type of targeted approach a “prodrug”—a medication designed to deliver its payload only in one area of the body.
DRP-104 (sirpiglenastat), a prodrug developed by Johns Hopkins, is currently in the early stages of clinical trials in people with advanced, strong tumors.The recently distributed examinations in mice show that the expanded medication specifically takes out disease cells but doesn’t hurt sound cells.
A report of the tests is distributed on November 16 in Science Advances.
“Our objective was to adjust an old malignant growth drug that had shown vigorous viability yet was excessively harmful, particularly to the stomach, to be grown clinically. To do this, we utilized a prodrug approach. What makes our methodology unique is that we used an original scientific plan to create a prodrug that was bio-enacted in disease cells but bio-inactivated in solid tissues like the stomach.”This specific targeting of the payload to malignant growth cells is now enabling this adequate class of medications to be rethought safely in individuals,” says focus on creator Barbara Slusher, Ph.D., M.A.S., head of the Johns Hopkins Medication Revelation Program and professor of nervous system science, pharmacology and subatomic sciences, psychiatry, neuroscience, medication, and oncology at the Johns Hopkins College Institute of Medication.
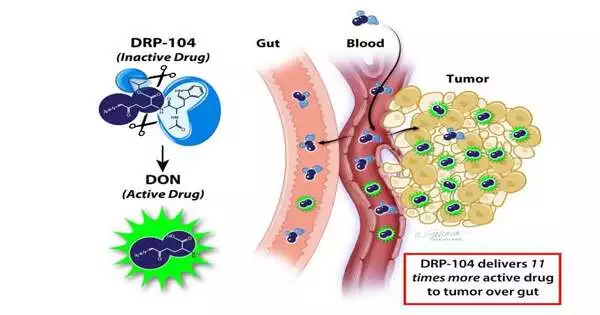
“DRP-104 is a tumor-targeted prodrug of DON (6-Diazo-5-Oxo-L-norleucine), a glutamine mimic drug that inhibits numerous glutamine-utilizing enzymes in cancer cells. Many early tests of DON revealed that it was highly effective in humans and animals, but its development was terminated due to its toxicity to normal tissues, particularly the stomach.”
Rana Rais, Ph.D., an associate professor of neurology and pharmacology,
The recently changed prodrug exploits a typical property of malignant growth cells: a ravenous hunger for an amino acid called glutamine, which is a basic structure block for proteins, lipids, and nucleotides, as well as concerning energy development. Quickly developing disease cells utilize an enormous measure of glutamine, a peculiarity called “glutamine enslavement.” Other solid cells with fast turnover, similar to those coating the stomach, likewise depend on glutamine.
Co-creator Rana Rais, Ph.D., an academic administrator of nervous system science and pharmacology, says, “DRP-104 is a growth-designated prodrug of the glutamine mirror drug called Wear (6-Diazo-5-Oxo-L-norleucine), which represses various glutamine-using proteins in disease cells.” Many early investigations of wear showed it was heartily solid in individuals and mice, yet its advancement was ended because of its poisonousness to typical tissues, particularly the stomach.
Improvement of this promising class of medications didn’t continue until Slusher, Rais, and their group chose to make compound alterations to Wear.
“We added synthetic gatherings, called promoieties, to Wear that delivered them latently in the body until they arrived at the growth, where the promoieties were cut off by chemicals that are bountiful in the growth but not in the stomach,” says Slusher, who is an individual from the Johns Hopkins Kimmel Disease Center and its BloombergKimmel Organization for Malignant Growth Immunotherapy. “This particular prodrug configuration caused Wear to be directed at its intended target (cancer) while also having an effect on solid cells elsewhere.”
For the new review, specialists gave the first Wear drug and the beefed-up DRP-104 medication to mice embedded with cancer. In mice that got DRP-104, the scientists found multiple times more dynamic medication in the cancerous tissues and the gastrointestinal tract (stomach). The two medications totally cleared out the growth, yet Wear caused more stomach poisoning in the mice than DRP-104.
Slusher and concentrate on co-creators Rana Rais, Pavel Majer, and Jonathan Powell, who helped establish a biotechnology organization, Dracen Drugs Inc., which authorized this new prodrug for clinical trials. DRP-104 is in stage I/II clinical preliminary testing at locales across the U.S., including the Johns Hopkins Kimmel Malignant Growth Place, for individuals with cutting-edge stage-severe cancers.
Slusher claims that her Johns Hopkins Medication Disclosure Lab is also actively looking for other medications that failed clinical trials due to toxicity issues. They desire to apply this equivalent-prodrug plan to medications for different circumstances.
More information: Rana Rais et al, Discovery of DRP-104, a tumor-targeted metabolic inhibitor prodrug, Science Advances (2022). DOI: 10.1126/sciadv.abq5925
Journal information: Science Advances