Azide intensifies and assumes a significant role in the ensuing blend of organonitrogens like amines and triazoles, which are fundamental mixtures in natural and materials science. Triazoles that can be blended by the ‘click’ response definitely stand out in the improvement of drugs and different enterprises. Be that as it may, the azido gatherings are electrophilic and are defenseless to different nucleophiles like carbanions. This represents a critical test for the blend of carbanions having azido gatherings.
To this end, a group of specialists from Japan, driven by Academic administrator Suguru Yoshida from Tokyo College of Science (TUS), has now fostered a creative and effective azide combination technique.
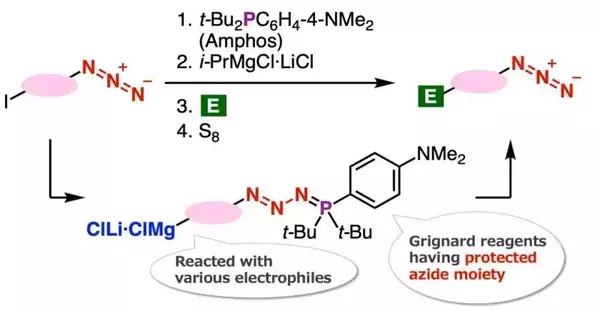
In their new article distributed in the journal Outskirts in Science, Dr. Yoshida and his associates, Ms. Rina Namioka (TUS) and Ms. Minori Suzuki (Tokyo Clinical and Dental College), have definite a productive strategy to plan organomagnesium (a natural compound containing a magnesium particle connected to a carbon) intermediates having a safeguarded azido gathering.
“We carried out this study because we think that by creating a synthesis process based on the azide group protection approach we found, the number of easily produced azide compounds can be increased.”
Associate Professor Suguru Yoshida from Tokyo University of Science (TUS),
“We directed this exploration since we accept that the scope of azide intensifies that can be effectively integrated can be extended by fostering a combination strategy in view of the azide bunch security technique that we found,” says Dr. Yoshida.
The curiosity of this combination strategy lies in the assurance of azido gatherings with di(tert-butyl)(4-(dimethylamino) phenylphosphine (Amphos) and following iodine-magnesium trade understood the arrangement of organomagnesium intermediates, which served in the amalgamation of assorted azides by changes with different electrophiles followed by deprotection with essential sulfur. In this review, the creators found another azide union strategy using the Grignard response and fostered another technique for the combination of 1,2,3-triazoles.
In particular, the group found that the iodine-magnesium trade response continues proficiently with aryl iodides having azido gatherings by utilizing the ‘azide bunch security technique’ that they have as of late evolved. Talking about the hidden instrument that empowered the analysts to accomplish this clever azide combination strategy, Dr. Yoshida reviews, “The Grignard response of organomagnesium intensifies that were combined by this response prevailed in the combination of an extensive variety of azide compounds.”
As azide intensifies and assumes a focal role in the combination of 1,2,3-triazoles in click science, this strategy is supposed to contribute to the effective improvement of drugs and other mechanically significant items.
“Our lab is at present leading examinations on the planning and change of carbanions with phosphazide moieties to extend the tool stash of such mixtures,” noticed a hopeful Dr. Yoshida. The consistent, effective combination of azides and the resulting union of a wide scope of organonitrogen mixtures like amines and triazoles will massively help engineer natural science, drug sciences, and materials science networks.
More information: Rina Namioka et al, Synthesis of 1,2,3-triazoles using Grignard reactions through the protection of azides, Frontiers in Chemistry (2023). DOI: 10.3389/fchem.2023.1237878