Specialists at EPFL have figured out how to concentrate on water in “a dead zone,” a freezing temperature range where water takes shape quickly. In the past, scientists have been unable to understand the strange properties of water because they couldn’t get to “no man’s land,” but a new method can now change that.
Water is one of the most important and common substances on Earth. It has shaped the planet’s composition and geology, controls its climate and weather patterns, and is the basis of all life as we know it. It covers more than 70% of the planet’s surface.
However, water is also odd. Over seventy different strange properties have been identified by scientists so far. A few speculations attempt to make sense of these inconsistencies, yet confirming them tentatively is troublesome. One reason is that this would require concentrating on water between 160 K and 232 K (-113 °C to -41 °C), an infamous temperature range known as “a dead zone,” where water takes shape so quickly that it has been beyond the realm of possibilities for researchers to concentrate on its properties.
“Our findings and method bring us one step closer to unraveling the mysteries of water. It’s tough to ignore the allure of this ubiquitous and seemingly simple liquid that has yet to reveal all of its secrets.”
Professor Ulrich Lorenz at EPFL’s School of Basic Sciences.
But why would anyone want to lower the temperature of water to such low levels? Since when water is cooled way beneath its edge of freezing, it becomes ‘supercooled’ with exceptional and intriguing properties; it can, for instance, remain liquid under certain conditions but instantly freeze when disturbed or exposed to certain substances. Supercooled water is made by cooling liquid water below the freezing point and using techniques to slow down or prevent crystallization of the water. But even with these ploys, crystallization in “no man’s land” still happens too quickly.
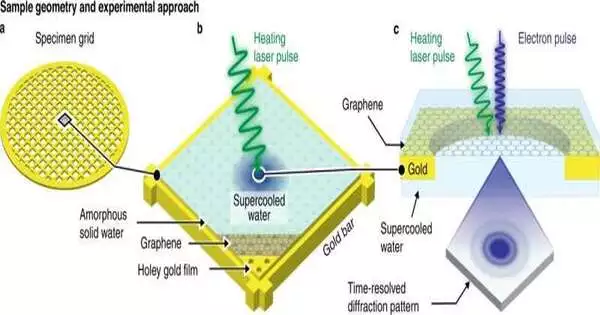
“An examination to efficiently test the design of water across a supposed “dead zone’ has stayed slippery for quite a long time,” says Teacher Ulrich Lorenz at EPFL’s School of Fundamental Sciences. That is exactly what scientists led by Lorenz have done recently. Before water can crystallize, the team devised a method for rapidly preparing deeply supercooled water at a precise temperature for electron diffraction analysis.
Lorenz asserts, “Despite this topic being hotly debated for over forty years, we have still not fully understood why water is an anomalous liquid.” “No man’s land” appears to hold the answer. But since quick crystallization, any estimation over the full temperature range has not been imaginable. This is our first attempt. We are getting closer to resolving this enduring mystery as a result of this.”
The scientists used a time-resolved electron microscope that they had made specifically for their lab to carry out the experiments. Before crystallization, they directly probed the supercooled water at a temperature that was clearly defined. They accomplished this by depositing a thin layer of amorphous ice and cooling a layer of graphene to 101 K. They then captured a diffraction pattern with an intense, high-brightness electron pulse and locally melted the film with a microsecond laser pulse in order to obtain water in “no man’s land.”
The researchers discovered that water’s structure changes smoothly as it cools from room temperature to cryogenic temperature. The structure of water begins to resemble that of amorphous ice—a type of ice in which water molecules are in a disordered state—at temperatures just below 200 K (approximately -73 °C). This is in contrast to the clean, crystalline ice that we are typically familiar with.
“The way that the design develops flawlessly permits us to limit the scope of potential clarifications for the beginning of water inconsistencies,” says Lorenz. “We are getting closer to unraveling the mysteries of water thanks to our findings and the method we have developed. It’s hard to avoid being captivated by this ubiquitous, seemingly straightforward liquid that has not yet revealed all of its secrets.
Nature Communications is the journal that published the research.
More information: Constantin R. Krüger et al, Electron diffraction of deeply supercooled water in no man’s land, Nature Communications (2023). DOI: 10.1038/s41467-023-38520-7