The world is profoundly dependent on petroleum products to drive its industry and transportation. These petroleum products lead to extreme carbon dioxide outflow, which adds to an unnatural weather change and sea fermentation. One method for lessening this extreme carbon dioxide outflow that is unsafe to the climate is through the electroreduction of carbon dioxide into readily added fillers or synthetics utilizing sustainable power. Utilizing this innovation to create methane has drawn wide interest. Nonetheless, analysts have had limited success in developing effective methane impetus.
A Soochow University research group has now fostered a basic system for making cobalt copper compound impetuses that convey remarkable methane action and selectivity in electrocatalytic carbon dioxide decrease. Their exploration is published in Nano Research.
In recent years, researchers have made significant progress in advancing understanding of how they might interpret impetuses and apply the information to their creation.Yet, the impetuses that have been created have not been good for use with methane, regarding selectivity or current thickness. In spite of the extraordinary experience researchers have acquired, the systems they have endeavored to make impetuses for methane are simply too expensive to ever be helpful in useful applications.
“Metal organic frameworks have been regarded as a distinct category of electrochemical carbon dioxide reduction reaction catalyst because they provide a tunable platform for systematically altering metal site coordination, regulating the Helmholtz layer, and exerting control over intermediates binding,”
Professor Yang Peng
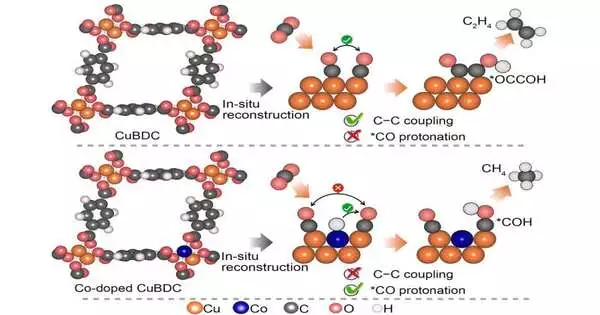
The Soochow University group focused on metal natural systems as a method for beating the prior challenges in building impetuses for methane. “The metal natural structures have been seen as a novel class of electrochemical carbon dioxide decrease response impetus since they offer a tunable stage to efficiently change the metal site coordination, direct the Helmholtz layer, and command over the intermediates restricting,” said Professor Yang Peng, Soochow Institute of Energy and Materials Innovations, College of Energy, Soochow University. The Helmholtz layer alludes to the limit or connection point that seems to be where an electronic guide interacts with an ionic guide.
However, the strength of metal natural systems during the electrolytic cycle remains a restricting issue. As a result, metal natural systems are frequently used as the primary forerunner to infer more powerful impetus groups upon remaking.In their examination, the group exploited the metal natural system’s homogenously scattered metal places. They achieved electrochemically decreased cobalt copper amalgams that convey remarkable methane action and selectivity in electrocatalytic carbon dioxide decrease. The group utilized in-situ X-beam adsorption spectroscopy and lessened all reflection surface degradation infrared spectroscopy in the advancement of their system.
The group’s review not just offers a helpful system for building electrocatalytic carbon dioxide decrease impetuses through the electrochemical remaking of bimetallic metal natural structures, but in addition, outfits significant experience in the guiding of electrocatalytic carbon dioxide decrease pathways on copper by means of nuclear doping of 3d change metals. These 3d change metals are the components on the occasional table running from 22Ti to 29Cu (titanium to copper).
By tweaking the cobalt doping focus, the group accomplished a striking Faradaic proficiency of 60% for methane at a high working current thickness.
“The main message we might want to convey in this work is that by molecularly doping other 3d change metals into copper, even in a little amount, the electrocatalytic carbon dioxide decrease energetics and pathway can be controllably tweaked,” said Peng.
In the following stage, the group needs to demonstrate better strength. They will do this by testing the reactant framework in a film cathode gathering. “Our definitive objective is to accomplish modern-scale efficiency and security of methane creation and understand the clever use of carbon dioxide in a green way,” said Peng.
More information: Hao Sun et al, Atomically dispersed Co−Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation, Nano Research (2022). DOI: 10.1007/s12274-022-4728-1
Journal information: Nano Research