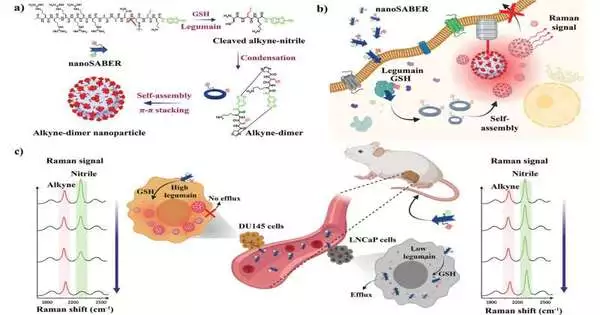
At the point when Jedi Knights need to vanquish an adversary, they whip out their dependable lightsabers. Later on, because of Johns Hopkins specialists, specialists trying to pulverize malignant growth might use microscopic sub-atomic nanoSABERs that permit them to check out cancers in manners never before conceivable.
Motivated by the interaction cells use to gather proteins, a group led by two specialists—Ishan Barman at the college’s Whiting School of Designing and Jeff W. Bulte, a teacher of radiology and radiological science at the Institute of Medication who is likewise partnered with JHU’s Establishment for NanoBioTechnology—has made minute tests that light up when they experience specific chemicals tracked down in disease cells. The capacity to picture growths completely and early could fundamentally upgrade disease imaging, illuminate treatment choices, and work on understanding results.
“This could be a distinct advantage for malignant growth treatment,” said Barman, an academic administrator of mechanical design at the Whiting School, of gathering biorthogonal protein acknowledgment (nanoSABER) tests. The group’s outcomes show up in cutting-edge science.
“The capacity of the probes to provide a detailed view at the molecular, cellular, and tissue levels provides a holistic perspective. Understanding what is truly happening at the tumor margins is critical to ensuring total cancer elimination and minimizing the odds of recurrence.”
Said lead author Swati Tanwar, a post-doctoral fellow in mechanical engineering.
Presently, tissue biopsies are the highest quality level for identifying most diseases; however, they can be estimated and even miss portions of growth hiding in the edges. The Johns Hopkins group’s methodology could take care of that issue, permitting clinicians to picture harmful movement across whole cancers, giving bits of knowledge into their conceivable forcefulness.
A 3D picture of nanoSABER in DU145 cells Credit: Johns Hopkins College
Proteins, particularly legumes, assume a main role in the turn of events and the movement of disease.
The group’s new device gathers itself within the sight of these disease-related catalysts and emanates a sign that can then be obtained by Raman spectroscopy, a representation strategy that investigates sub-atomic vibrations to recognize and portray substances. This permits the tests to precisely pinpoint disease cells.
The Johns Hopkins group says its technique likewise could permit clinicians to all the more precisely screen the aggregation of disease drugs in cancers during therapy, giving a sign of how well those therapies are functioning.
“The tests’ capacity to offer a reasonable gander at the sub-atomic, cell, and tissue levels gives a complete viewpoint,” said lead creator Swati Tanwar, a post-doctoral individual in mechanical design. “It is basic to comprehend what is truly occurring at the growth edges to guarantee total disease evacuation and limit the possibilities of repeat.”
Concentrate on co-creators at Johns Hopkins, including Behnaz Ghaemi, Piyush Raj, Aruna Singh, Lintong Wu, Dian R. Arifin, and Michael T. McMahon. The group additionally included Yue Yuan of the College of Science and Innovation of China.
More information: Swati Tanwar et al, A Smart Intracellular Self-Assembling Bioorthogonal Raman Active Nanoprobe for Targeted Tumor Imaging, Advanced Science (2023). DOI: 10.1002/advs.202304164