An inexpensive nanomaterial has been shown to be capable of removing industrial emissions of carbon dioxide by researchers in the College of Science at Oregon State University.
The discoveries, distributed in Cell Reports Actual Science, are significant on the grounds that better carbon capture strategies are a vital aspect of tending to environmental change, said OSU’s Kyriakos Stylianou, who drove the review.
Carbon dioxide, an ozone-depleting substance, comes about because of the consumption of petroleum products and is one of the essential drivers of a warming environment.
“Carbon dioxide capture is critical for meeting net-zero emission targets, MOFs have shown great promise for carbon capture due to their porosity and structural versatility, but synthesizing them often necessitates the use of reagents that are both economically and environmentally costly, such as heavy metal salts and toxic solvents.”
Stylianou, an assistant professor of chemistry.
Stylianou points out that although facilities that remove carbon from the air are beginning to appear all over the world—the largest of their kind opened in Iceland in 2021—they are not yet equipped to significantly address the issue of global emissions. The Iceland plant is able to remove enough carbon dioxide in a year to offset about 800 cars’ annual emissions.
Notwithstanding, innovations for moderating carbon dioxide at the place of passage into the climate, like a production line, are similarly advanced. Metal organic frameworks, or MOFs, which are nanomaterials that are capable of adsorbing carbon dioxide molecules as flue gases pass through smokestacks, are one of these technologies.
Stylianou, an assistant professor of chemistry, stated, “For meeting net-zero emission targets, the capture of carbon dioxide is critical.” Due to their porosity and structural adaptability, MOFs have shown a lot of promise for carbon capture. However, synthesizing them frequently requires the use of reagents that are expensive both economically and environmentally, like toxic solvents and heavy metal salts.”
He also stated that dealing with the water component of smokestack gases makes removing carbon dioxide extremely challenging. Numerous MOFs that have shown carbon capture have lost their viability in damp conditions. Stylianou stated that flue gases can be dried, but doing so significantly increases the cost of the carbon dioxide removal process, making it unsuitable for industrial applications.
“As a result, we attempted to develop a MOF to address the various limitations of the materials used in carbon capture at the present time: low CO2 uptake capacities, low stability in humid conditions, high cost, and poor selectivity for carbon dioxide,” he stated.
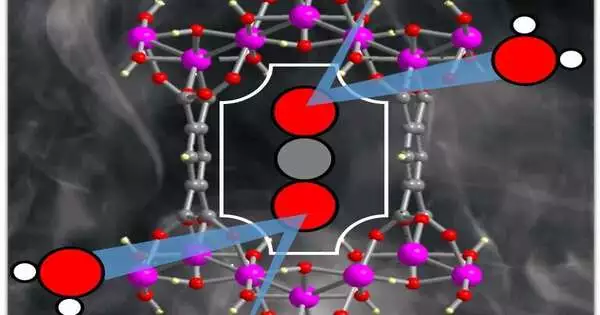
MOFs are translucent, permeable materials comprised of decidedly charged metal particles encompassed by natural “linker” atoms known as ligands. Nodes are formed by the metal ions, which bind the arms of the linkers to form a cage-like structure that repeats itself. Similar to a sponge, the structure has nanosized pores that adsorb gases.
MOFs can be planned with various parts that decide the MOF’s properties, and there are a great many conceivable MOFs, Stylianou said. Chemistry researchers have synthesized nearly 100,000 of them and predicted the properties of another half a million.
Stylianou stated, “In this study, we introduce a MOF composed of aluminum and a readily available ligand, benzene-1,2,4,5-tetracarboxylic acid.” The combination of the MOFs occurs in water and only requires several hours. Furthermore, the MOF has pores with a size similar to that of CO2 particles, meaning there’s a bound space for imprisoning the carbon dioxide.”
The MOF functions admirably in clammy circumstances and furthermore favors carbon dioxide over nitrogen, which is significant on the grounds that nitrogen oxides are a fixing in vent gasses. The MOF could potentially be binding to the wrong molecules without that selectivity.
According to Stylianou, “This MOF is an outstanding candidate for wet post-combustion carbon capture applications.” It can be regenerated and reused at least three times with comparable uptake capacities, is cost-effective, and has exceptional separation performance.
Along with Oregon State chemists Ryan Loughran, Tara Hurley, and Andrzej Gadysiak, researchers from Columbia University, the Pacific Northwest National Laboratory, and Chemspeed Technologies AG in Switzerland also participated in this study.
More information: Ryan P. Loughran et al, CO2 capture from wet flue gas using a water-stable and cost-effective metal-organic framework, Cell Reports Physical Science (2023). DOI: 10.1016/j.xcrp.2023.101470