Rice University synthetic specialists have worked on their plan for a light-controlled impetus that quickly separates PFOA, one of the world’s generally risky “perpetually compound” poisons.
In 2020, Michael Wong and his understudies made the astounding revelation that boron nitride, a financially accessible powder that is normally utilized in beauty care products, could obliterate the vast majority of PFOA, or perfluorooctanoic corrosive, in water tests inside only a couple of hours when it was presented to bright light with a frequency of 254 nanometers.
“That was extraordinary on the grounds that PFOA is an undeniably tricky contamination that is truly difficult to obliterate,” said Wong, relating the creator of the overhauled impetus in Chemical Engineering Journal. Yet, it was likewise not great on the grounds that the boron nitride was initiated by short-wave UV, and the climate sifts through practically all of the short-wave UV of daylight. We needed to push however much as could be expected of boron nitride’s capacity to get energy from different frequencies of daylight. “
Long-wave UV, or UV-A, has frequencies going from around 315-400 nanometers. It causes suntans and burns from the sun, and ample daylight arrives on Earth. Boron nitride is a semiconductor, and it isn’t enacted by UV-A. Titanium dioxide, a typical fixing in sunscreen, is a semiconductor that is enacted by UV-A, and it has even been displayed to catalyze the breakdown of PFOA, though leisurely, when presented to UV-A.
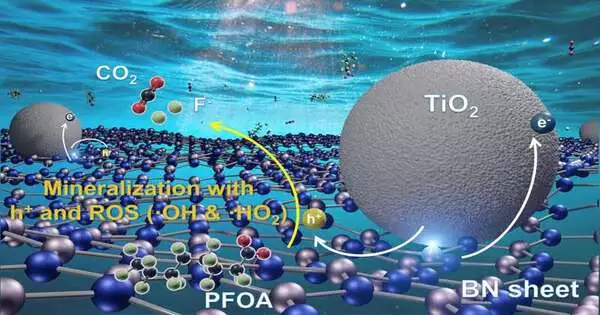
So Wong and co-lead creators Bo Wang, Lijie Duan, and Kimberly Heck decided to create a composite of boron nitride and titanium dioxide that combined the best aspects of the individual impetuses.In their new review, they showed the UV-A controlled composites obliterated PFOA multiple times quicker than plain titanium dioxide photocatalysts.
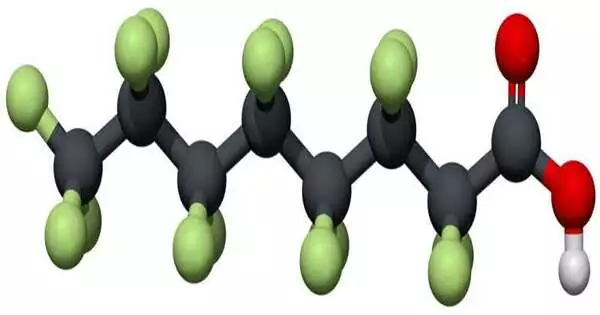
Atomic construction of perfluorooctanoic corrosive, or PFOA, one of the world’s generally pervasive “perpetually synthetic” toxins.
By dissecting photocurrent reaction estimations and different information, Wong’s group figured out how its semiconductor composite collected UV-An energy to fall to pieces PFOA particles in water. In open-air tests utilizing plastic water bottles under normal daylight, they found the boron nitride-titanium dioxide composites could corrupt the vast majority of PFOA in deionized water in under three hours. In pungent water, that cycle requires around nine hours.
Mounting proof suggests PFOA is hurtful to human wellbeing. Some U.S. states have drawn certain lines on PFOA defilement in drinking water, and in March 2021, the Environmental Protection Agency reported plans to foster government principles.
Developing administrative strain to set PFOA principles has water treatment plants searching for new and cost-proficient approaches to eliminate PFOA from water, Wong said.
PFOA is one of the most pervasive PFAS (perfluoroalkyl and polyfluoroalkyl substances), a group of mixtures created in the twentieth century to make coatings for waterproof clothing, food bundling, and different items. PFAS have been named synthetic substances since they aren’t effectively corrupted and will generally wait in the climate. Wong said his group is surveying how well its composite photocatalyst functions for separating other PFAS.
He said the boron nitride and composite impetus advances stand out from a few modern accomplices in the Rice-based Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (NEWT), which is supported by the National Science Foundation to foster off-matrix water treatment frameworks.
More information: Lijie Duan et al, Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid, Chemical Engineering Journal (2022). DOI: 10.1016/j.cej.2022.137735