A new study adds to the growing body of evidence that organoids – lab-grown collections of cells that mimic a patient’s tumor – are a promising avenue for drug discovery to improve outcomes in cancer patients, particularly in rare cancers where clinical data on drug effectiveness is frequently lacking.
Researchers at the University of California, Los Angeles (CA, USA) have refined a platform for growing tumor replicas – known as tumor organoids – using cells biopsied from a patient’s tumor. Using these organoids, the team was able to find potential therapy choices for five patients who had few options prior.
Organoids are created in a lab using a patient’s own tissue cells acquired during surgery. These “mini tumors” are simplified, smaller copies of biological organs or tumors that reproduce full-function components. Dr. Alice Soragni’s laboratory at UCLA pioneered their development and use to the study of diseases and potential remedies.
In the most recent study, published online in Science Advances, researchers led by Dr. Soragni acquired seven tumor samples from five patients with chordoma, a rare bone cancer with few treatment choices. Chordomas are tumors that develop in the sacrum or at the base of the skull. Surgery is their primary treatment, however due to their position, total excision is not always possible, and recurrence rates are high. Chordomas do not respond to standard chemotherapy, and their rarity (about one instance per million individuals in the United States every year) hinders studies to find viable medicines.
Our research continues to focus on developing personalized models for uncommon malignancies, which frequently lack therapeutic choices, with the long-term goal of translating these findings into clinical trials.
Dr. Alice Soragni
“There is an urgent need for clinically relevant, tailored models to discover therapies for chordoma and many other uncommon malignancies,” said Soragni, an assistant professor in the Department of Orthopaedic Surgery and a member of the Jonsson Comprehensive Cancer Center. “Because this cancer is so uncommon, and there are so few models available, our capacity to research how it responds to possible therapies is severely constrained. We optimized a platform for growing organoids from tumor cells in order to examine their biological properties and establish which pathways could be most promising for treatment by testing them against a wide range of treatments.”
According to the current study, researchers were able to effectively produce viable organoids from all samples. The patient-derived organoids had morphologies and characteristics that were similar to the actual chordoma tumors from which they were derived, such as expressing a protein called Ki-67, which is associated with cell proliferation, and brachyury, a protein that is a well-established marker of chordoma.
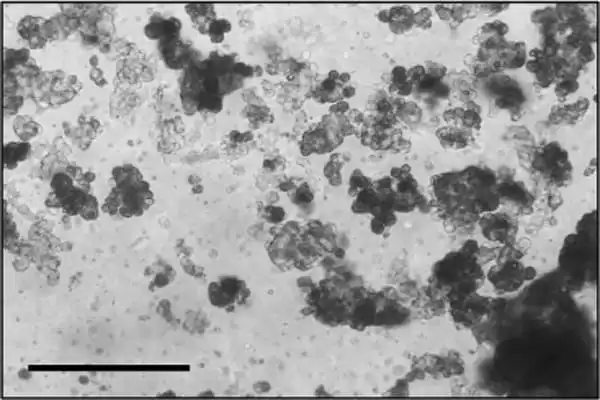
The organoids were then utilized to do high-throughput drug screening, an automated drug discovery approach that allows a huge number of possible therapies to be examined at once rather than one at a time, considerably speeding up the development and testing of prospective targets and drugs. This type of large-scale screening has been critical in medication development and testing, but this is the first time it has been applied to patient-derived chordoma organoids.
The scientists discovered many pharmacological targets and biological pathways that might potentially be pursued for chordoma therapy as a result of the screening. They also emphasize the significance of a tailored strategy, owing to the disparities in responses reported among individuals as well as between tumors retrieved from the same patient.
“We’ve demonstrated that the patient-derived tumor organoids we develop can be effectively screened against hundreds of drugs using our platform, which now includes machine learning approaches to study organoids growth patterns and pathway analysis to find targetable biological processes,” Soragni said. Dr. Soragni’s laboratory has expanded its screening capacity over the last year, allowing it to develop and test hundreds to thousands of medications on tumor organoids each week, thanks to monies from an NIH grant and the Department of Orthopaedic Surgery.
“Our automation-compatible strategy of producing and screening patient-derived tumor organoids has been shown to be effective for uncommon carcinomas (Phan et al, 2019) and, more recently, slow-growing bone cancers. Our research continues to focus on developing personalized models for uncommon malignancies, which frequently lack therapeutic choices, with the long-term goal of translating these findings into clinical trials.”
Interestingly, despite having the same uncommon malignancy, each patient had different findings, according to the study. Furthermore, the study discovered that two tumors from the same patient had different outcomes. “Our work continues to be focused on building customized models for uncommon malignancies, which often lack therapeutic alternatives,” lab leader Alice Soragni said, “with a long-term goal to utilize these discoveries in the clinic.”
This is the first study to use high-throughput drug screening methodologies to patient-derived organoids, and it offers a compelling case for personalized medicine’s future in cancer research.