While leading a generally clear examination concerning the gathering component of calcium-phosphate bunches, specialists at UC Santa Barbara and New York University made an astonishing revelation: phosphate particles in water have an inquisitive propensity for suddenly switching back and forth between their normally experienced hydrated state and a secretive, beforehand unreported “dull” state.
This recently discovered behavior, they claim, has implications for understanding the role of phosphate species in biocatalysis, cell energy balance, and biomaterial arrangement.Their discoveries are documented in the Public Foundation of Sciences Procedures.
“Phosphate is all over,” said UCSB science teacher Songi Han, one of the creators of a paper in the Procedures of the Public Foundation of Sciences. The particle consists of one phosphorus iota encompassed by four oxygen molecules. “It’s in our blood and in our serum,” Han continued. “It’s in each researcher’s cradle; it’s on our DNA and RNA.” “It’s likewise a primary part of our bones and cell layers,” she added.
When phosphates are bound with calcium, they form small, subatomic groups on their way to forming mineral stores in cells and bones.That is exactly what Han and colleagues Matthew Helgeson at UCSB and Alexej Jerschow at NYU planned to review and depict in order to reveal quantum behaviors in symmetric phosphate groups proposed by UCSB physical science teacher Matthew Fisher.On the whole, the specialists needed to set up control tests, which included sweeps of phosphate particles without calcium through atomic attractive reverberation (NMR) spectroscopy and cryogenic transmission electron microscopy (cryo-TEM).
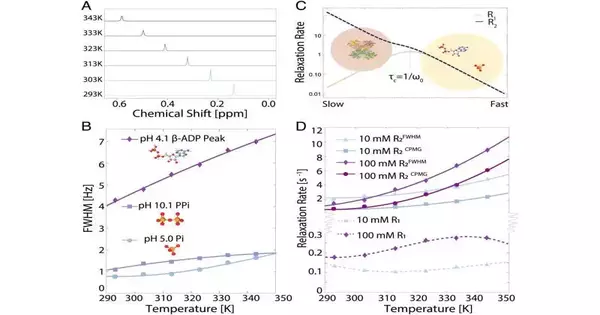
However, while the UCSB and NYU understudies on the task were gathering reference data, which included the naturally occurring isotope phosphorus 31 in watery arrangements at various focuses and temperatures, their outcomes did not coordinate with assumptions.For example, Han stated that the line addressing the range for 31P during NMR examinations should limit as temperatures rise.
“The explanation is that, as you go to higher temperatures, the particles tumble quicker,” she made sense of. Commonly, this quick atomic movement would average out the anisotropic cooperations, or communications, that are subject to the general directions of these little particles. The outcome would be a restriction of resonances estimated by the NMR instrument.
“We were expecting a phosphorus NMR signal, which is a straightforward one, with a pinnacle in that river with higher temperatures,” she said. “Shockingly, however, we estimated spectra that were widening, doing the direct inverse of what we anticipated.”
This bizarre outcome shifted the group’s focus, prompting a flurry of investigations to determine the cause at the subatomic level.The decision, after a period of avoiding an endless series of speculations?Phosphate particles were forming bunches under many organic circumstances—groups that were sidestepping direct spectroscopic recognition, which is reasonable given why they had not been seen previously. Moreover, the estimations recommended that these particles were switching back and forth between a noticeable “free” state and a dim “collected” state, resulting in the expansion of the sign rather than a sharp pinnacle.
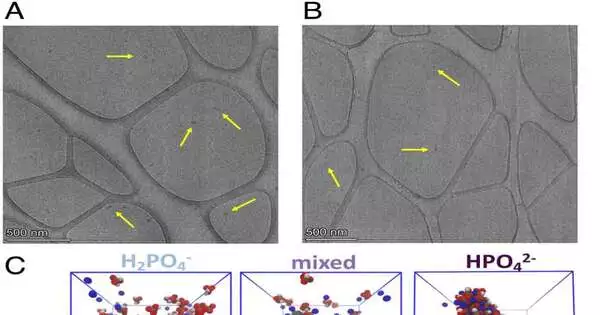
Proof of phosphate gatherings from TEM and MD reenactments (A and B) TEM pictures of phosphate gatherings (yellow bolts) subsequent to warming phosphate arrangements show drop-like elements framing at 25 to 50 nm in size. The tests came from various sources and were completed on various days.(A) 100 mM potassium ADP warmed to 343 K before vitrification. (B) 100 mM sodium ADP warmed to 343 K before vitrification. (C) MD recreation group size dispersions at 343 K show the part, P(N), of phosphate particles in a bunch of size Nclust. The insets show previews of phosphate aggregates (red and white) and sodium particles (blue) from the recreations. The group size appropriation and previews show that HPO42 collects more emphatically than H2PO4.At the point when H2PO4 is blended in with HPO42, the last option activates the grouping of H2PO4. In this blended framework, the HPO42 particles are turned gray to feature the grouping of H2PO4. Visual Atomic Elements are used to create recreation previews.
Furthermore, as the temperature rose, the number of these gathered states increased, indicating yet another temperature-dependent behavior, according to co-lead creator Mesopotamia Nowotarski.
“The end result of those trials was that the phosphates are drying out, which allows them to draw closer together,” she explained.At lower temperatures, by far most of these phosphates in this arrangement grip water particles that structure a defensive water coat around them. This hydrated state is commonly expected when thinking about how phosphate acts in organic frameworks.
Be that as it may, at higher temperatures, Nowotarski made sense of it; they shed their water shields, permitting them to adhere to one another. This idea was affirmed by NMR tests that examined the phosphate water shell and further approved by an investigation of cryo-TEM pictures to recognize the presence of bunches as well as displaying the energetics of phosphate gathering by co-lead creator Joshua Straub.
These unique phosphate congregations and hydration shells have significant ramifications for science and natural chemistry, as per the scientists. Phosphate, said synthetic specialist Matthew Helgeson, is an ordinarily grasped “money” utilized in organic frameworks to store and consume energy through change into adenosine triphosphate (ATP) and adenosine diphosphate (ADP).
“Assuming hydrated phosphate, ADP, and ATP address little “bills” of cash, this new revelation recommends that these more modest monetary standards can trade with a lot bigger divisions, say $100, which might have totally different connections with biochemical cycles than presently known components,” he said.
Additionally, numerous biomolecular parts incorporate phosphate bunches that may, similarly, form structure groups. Following that, the discovery that these phosphates can unexpectedly aggregate could provide some insight into other fundamental organic cycles such as biomineralization—how shells and skeletons structure, as well as protein connections.
“We likewise tried a scope of phosphates, including those integrated into the ATP particle, and they all seemed to show a similar peculiarity, and we accomplished quantitative examination for these gatherings,” said co-lead writer Jiaqi Lu.
This previously unnoticed cycle could also be significant in the domains of cell flagging, digestion, and disease cycles like Alzheimer’s disease, where the connection of a phosphate gathering, or phosphorylation, to the protein tau in our mind is normally found in neurofibrillary tangles—a sign of neurodegeneration.Having observed and concentrated on this gathering behavior, the group is now delving deeper, with concentrations on the impact of pH on phosphate gathering, hereditary interpretation, and adjusted protein gathering, in addition to their unique work on calcium phosphate gathering.
“It truly impacts the manner in which we contemplate the job of phosphate bunches; we commonly don’t think about a driver of sub-atomic get-togethers,” Han said.
More information: Joshua S. Straub et al, Phosphates form spectroscopically dark state assemblies in common aqueous solutions, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2206765120
Journal information: Proceedings of the National Academy of Sciences