Johns Hopkins scientists, alongside partners in Italy, have distributed a concentrate in Science Advances that investigates the hereditary systems behind the improvement of schizophrenia.
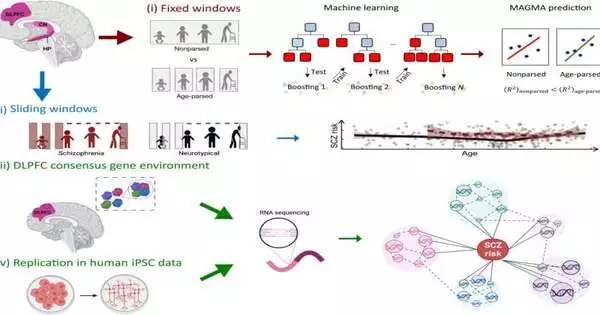
The paper, “Agreement sub-atomic climate of schizophrenia risk qualities in coexpression networks moving across age and cerebrum locales,” centers around the union of schizophrenia risk qualities in mind coexpression networks in the after-death human prefrontal cortex, hippocampus, caudate core, and dentate gyrus granule cells, assembled by unambiguous age sections in 833 examples, 186 of which were obtained from schizophrenia-analyzed patients.
The outcomes support an early contribution in the prefrontal cortex with quality articulation that marks basic schizophrenia and uncover a powerful transaction of locales wherein age organizing makes sense of more change in schizophrenia risk compared with dissecting all age sections together.
“In coexpression networks that change with age and brain regions, schizophrenia risk genes have a consensus molecular environment.”
Johns Hopkins researchers,
The hereditary engineering of schizophrenia was found to incorporate moving coexpressed quality set designs across cerebrum districts and time, possibly making sense of moving clinical introductions in patients. In particular, there was a huge combination of chance qualities communicated in the dorsolateral prefrontal cortex of adolescents.
The dorsolateral prefrontal cortex (DLPFC) is a region of the cerebrum where numerous fundamental exercises happen, for example, dynamic, cognizant direction, working memory, result expectations, drive hindrance, and contemplated considerations—aall in all known as leader capabilities. Additionally, it is a significant hub in the mental choice of tactile data.
Utilizing past examinations to recognize 28 qualities reliably found cooperating in modules improved for schizophrenia risk qualities in DLPFC, analysts found 23 recently kept in schizophrenia concentrates with an unidentified relationship with risk qualities. These co-communicated qualities might have evaded past analysts due to the age-subordinate way they are communicated.
A model given in the paper represents how schizophrenia risk modules enhanced for a record factor, focused on in the hereditary relationship with schizophrenia, are found in the perinatal and adolescent DLPFC yet not later, thus disappearing from information that isn’t age explicit.
Analysts report that the most surprising and novel aftereffect of the review is the recognizable proof of change and bigoted agreement qualities that are particularly liable to be coexpressed with schizophrenia risk qualities. This arrangement of qualities was coexpressed with risk qualities in many organizations analyzed, paying little heed to contrasts across datasets, preprocessing pipelines, and boundary settings.
While consolidating the information of modules containing these agreement qualities, the outcomes showed solid enhancement for realized schizophrenia risk qualities, which was not found when combining modules without agreement qualities. The quality ontologies for the agreement quality set and their modules underscored neurons, neurotransmitters, and particle transport. From a chromosomal view, these agreement qualities are not bunched, precluding locus-related curiosities.
The clever manner in which the review set off to investigate the development of schizophrenia by age bunches permitted the analysts to notice relationships that were available but missed in past examinations. Having a superior viewpoint of normally coexpressed qualities gives a more complete image of the sub-atomic climate in which the illness is occurring and draws us closer to distinguishing the components at work. It could likewise assist clinicians with better comprehension of the divergent signs they saw in patients after some time.
More information: Giulio Pergola et al, Consensus molecular environment of schizophrenia risk genes in coexpression networks shifting across age and brain regions, Science Advances (2023). DOI: 10.1126/sciadv.ade2812