Scientists at the College of Arizona Disease Center found another class of iron-focusing intensifies that hamper the expansion of refined dangerous cells in a lab setting. The consequences of the review were distributed in the Diary of the American Compound Society.
“Disease cells are what we call ‘dependent on’ iron, so we are making intensifies that can disrupt the accessibility of iron in malignant growth cells,” said Elisa Tomat, Ph.D., teacher in the Branch of Science and Natural Chemistry at the UArizona School of Science and individual from the UArizona Disease Center.
The revelation could prompt the advancement of a wide range of anticancer medications that target iron digestion.
The group has been working with Tech Send Off Arizona, the college’s commercialization arm, fully intent on permitting the innovation to an organization that will move it into the commercial center. A patent application is forthcoming.
Iron is the most plentiful change metal in the human body and, as per Tomat, assumes a significant role in growth, movement, and metastasis. Disease cells depend on a few iron-subordinate cycles to support their quick multiplication rates and hence have a higher popularity for this component compared to ordinary cells.
Tomat said the exploration group’s test was catching iron inside threatening cells at this point, keeping it accessible to the remainder of the body. To do so, they designated intracellular iron with intensifies that are enacted solely after cell take-up.
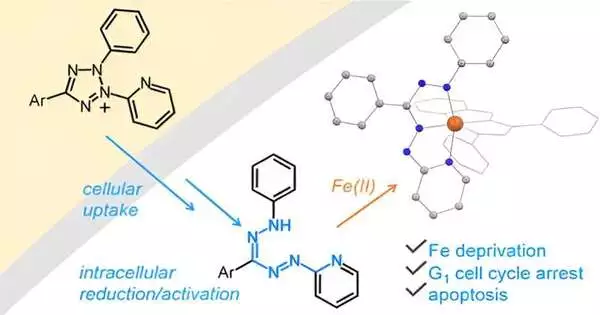
Graphical theoretical. Credit: Diary of the American Substance Society (2023). DOI: 10.1021/jacs.3c02033
“As scientists, we can plan and blend atoms that can tie iron together just under specific circumstances and not all through the body,” Tomat said. “We’ve been chipping away at different methodologies toward this sort of science; we call these prochelator approaches in light of the fact that the chelator is the compound that ties the metal particle. The prochelator is the compound we intended to become enacted exclusively after going through a specific response that happens in cells.”
The exploration was propelled by a “typical reagent,” a compound that is utilized in research facilities overall to survey the capacity of medication to restrain the multiplication of refined mammalian cells.
“Since iron is such a crucial player that is significant in numerous disease types, and this popularity for iron is an overall trait of threat, I’ve been keen on this technique for various years,” said Tomat, who has been investigating iron chelators and their job in growth movement for over 10 years.
“We’re amped up for this new technique since we figure this class of particles can be additionally changed to enhance the properties, work on the antiproliferative action, and truly become a way for us to influence the iron accessibility in dangerous cells and stop disease development.”
Tomat’s co-creators incorporate his previous postdoctoral partner, Zoufeng Xu, Ph.D., and doctoral understudy Yu-Shien Sung.
More information: Zoufeng Xu et al, Design of Tetrazolium Cations for the Release of Antiproliferative Formazan Chelators in Mammalian Cells, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c02033





