Scientists at NYU Tandon School of Designing accomplished a significant leap forward in Redox Stream Desalination (RFD), an emerging electrochemical strategy that can transform seawater into consumable drinking water and furthermore store reasonable sustainable power.
In a paper distributed in Cell Reports Actual Science, the NYU Tandon group, led by Dr. André Taylor, a teacher of compound and biomolecular design and overseer of DC-Dream (Decarbonizing Substance Assembling Utilizing Reasonable Jolt), expanded the RFD framework’s salt evacuation rate by roughly 20% while bringing down its energy interest by streamlining liquid stream rates.
RFD offers numerous advantages. These frameworks provide a versatile and adaptable way to deal with energy capacity, empowering the productive use of discontinuous sustainable power sources, for example, solar and wind. RFD likewise guarantees an altogether new answer for the worldwide water emergency.
“Our goal is to develop a sustainable and effective solution that not only satisfies the increasing demand for freshwater but also promotes environmental preservation and the integration of renewable energy sources by seamlessly combining energy storage and desalination.”
Dr. André Taylor, professor of chemical and biomolecular engineering and director of DC-MUSE.
“Via flawlessly coordinating energy stockpiling and desalination, our vision is to make a manageable and productive arrangement that fulfills the developing need for freshwater as well as champions ecological preservation and environmentally friendly power combinations,” said Taylor.
RFD can both diminish dependence on customary power networks and furthermore encourage the change towards a carbon-nonpartisan and eco-accommodating water desalination process. Besides, the mix of redox stream batteries with desalination advancements upgrades framework proficiency and unwavering quality.
The intrinsic capacity of redox stream batteries to store excess energy during times of overflow and release it during peak interest adjusts consistently with the fluctuating energy necessities of desalination processes.
“The progress of this undertaking is credited to the inventiveness and determination of Stephen Akwei Maclean, the paper’s most memorable creator and a NYU Tandon Ph.D. competitor in synthetic and biomolecular designing,” said Taylor. “He exhibited outstanding expertise by planning the framework engineering utilizing progressed 3D printing innovation accessible at the NYU Creator Space.”
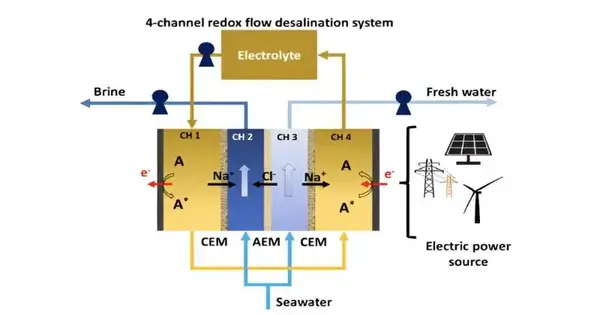
The complexities of the framework include the division of approaching seawater into two streams: the salinating stream (see picture above, CH 2) and the desalinating stream (see picture above, CH 3). Two extra channels house the electrolyte and redox particles (picture over, A). These channels are successfully isolated by either a cation trade layer (CEM) or an anion trade film (AEM).
In CH 4, electrons are provided from the cathode to the redox atom, removing Na+ that diffuses from CH 3. The redox atom and Na+ are then shipped to CH 4, where electrons are provided to the anode from the redox particles, and Na+ is permitted to diffuse into CH 2. Under this general potential, Cl-particles move from CH 3 through the AEM to CH 2, framing the concentrated saline solution stream. Thus, CH 3 produces the freshwater stream.
“We have some control over the approaching seawater home opportunity to deliver drinkable water by working the framework in a solitary pass or group mode,” said Maclean.
More information: Stephen A. Maclean et al. Investigation of flow rate in a symmetric four-channel redox flow desalination system, Cell Reports Physical Science (2024). DOI: 10.1016/j.xcrp.2023.101761