Seawater is essential to life on Earth due to its composition of hydrogen, oxygen, sodium, and other elements. But because of the same intricate chemistry, it is challenging to extract hydrogen gas for use in clean energy applications.
Researchers at Stanford University, the Department of Energy’s SLAC National Accelerator Laboratory, and their partners at the University of Oregon and Manchester Metropolitan University have now discovered a method to draw hydrogen from the ocean by passing seawater through a double-membrane system and electricity. Their ground-breaking method has succeeded in producing hydrogen gas without creating a significant amount of harmful byproducts. Their study’s findings, which were released today in Joule, may aid efforts to produce low-carbon fuels.
“Many water-to-hydrogen systems today attempt to use a monolayer or single-layer membrane. Adam Nielander, an associate staff scientist at the SUNCAT Center for Interface Science and Catalysis, a SLAC-Stanford joint institute, explained how his study combined two layers. “In our experiment, we were able to regulate the movement of ions in seawater thanks to these membrane architectures.”.
“A monolayer or single-layer membrane is used in many water-to-hydrogen systems today. Our research combined two levels. In our experiment, we were able to control the movement of ions in seawater thanks to these membrane structures.”
Adam Nielander, an associate staff scientist with the SUNCAT Center for Interface Science and Catalysis.
An option for long-duration energy storage for electric grids, hydrogen gas is a low-carbon fuel that is currently used in a variety of applications, including the operation of fuel-cell electric vehicles.
Fresh or desalinated water is a common starting point in many attempts to produce hydrogen gas, but those processes can be costly and energy-intensive. Less substances—chemical elements or molecules—float around in treated water, making it simpler to work with. However, the researchers noted that water purification is costly, energy-intensive, and increases device complexity. They also suggested using natural freshwater, which is more scarce on the planet and also has some impurities that are problematic for contemporary technology.
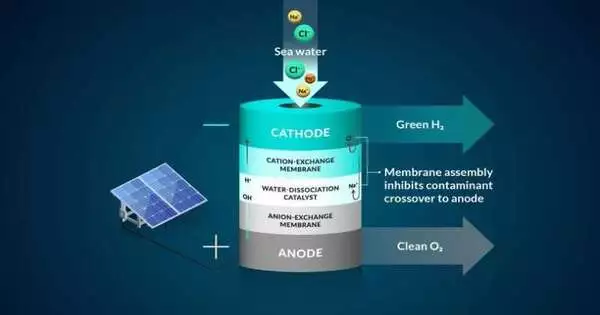
The group developed a bipolar (two-layer) membrane system for use with seawater and tested it using electrolysis, a process that uses electricity to drive ions, or charged particles, to run a desired reaction. According to Joseph Perryman, a postdoctoral researcher at SLAC and Stanford, they began their design by regulating chloride, the most harmful element in the seawater system.
According to Perryman, sodium chloride, which contributes significantly to seawater’s salinity, is one of the many reactive species in seawater that can obstruct the water-to-hydrogen reaction. “In particular, chloride that reaches the anode and oxidizes will shorten the lifespan of an electrolysis system and may even become dangerous because of the toxic nature of the byproducts of oxidation, which include molecular chlorine and bleach.”.
In the experiment, the bipolar membrane prevents chloride from entering the reaction center while permitting access to the conditions required to produce hydrogen gas.
“We are effectively using two different strategies to halt this chloride reaction,” Perryman said.
A place for hydrogen.
An ideal membrane system accomplishes three main tasks: it separates hydrogen and oxygen gases from seawater, it aids in moving only the beneficial hydrogen and hydroxide ions while restricting other seawater ions, and it aids in preventing undesirable reactions. Because it is challenging to capture all three of these functions simultaneously, the team’s research focuses on investigating systems that can successfully combine all three of these requirements.
In particular, protons, which were the positive hydrogen ions in their experiment, passed through one of the membrane layers to a location where they could be gathered and converted into hydrogen gas by interacting with a negatively charged electrode (cathode). Only negative ions, like chloride, were able to pass through the second membrane in the system.
As an additional safeguard, one membrane layer contained negatively charged groups that were fixed to the membrane, which made it more difficult for other negatively charged ions, like chloride, to move to locations where they shouldn’t be, according to Daniela Marin, a Stanford graduate student in chemical engineering and co-author. The team’s experiments showed the negatively charged membrane to be extremely effective at obstructing almost all chloride ions, and their system functioned without producing harmful byproducts like chlorine or bleach.
The study improved their overall knowledge of how seawater ions moved through membranes and helped them design a seawater-to-hydrogen membrane system, according to the researchers. This information could aid in the development of stronger membranes for use in other processes, such as the production of oxygen gas.
In order to create oxygen, electrolysis is also being considered, according to Marin. For this effort, it is also essential to comprehend how ions flow through and convert in our bipolar membrane system. In our experiment, we demonstrated how to generate oxygen gas using the bipolar membrane in addition to producing hydrogen.
The team’s next goal is to develop better electrodes and membranes using more widely available and accessible raw materials. According to the team, this design modification may make it simpler to scale up the electrolysis system to produce hydrogen for energy-intensive industries like the transportation sector.
The scientists also intend to transport their electrolysis cells to the Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC so that they can use the facility’s potent X-rays to examine the atomic structure of membranes and catalysts.
Thomas Jaramillo, professor at SLAC and Stanford and head of SUNCAT, declared that “green hydrogen technologies have a promising future.”. “The fundamental understandings we are gaining are crucial for guiding future innovations for better performance, durability, and scalability of this technology.”.
More information: Daniela H. Marin et al, Hydrogen production with seawater-resilient bipolar membrane electrolyzers, Joule (2023). DOI: 10.1016/j.joule.2023.03.005