In another review, specialists from the College of Copenhagen utilized light and chlorine to annihilate low-focus methane from the air. The outcome draws us closer to having the option to eliminate ozone-harming substances from animal lodging, biogas creation plants, and wastewater treatment plants to help the environment. The work has been accepted for publication in Environmental Research Letters.
The Intergovernmental Board on Environmental Change (IPCC) has confirmed that lessening methane gas emanations will promptly decrease the climb in worldwide temperatures. The gas is 85 times more potent than CO2 as a greenhouse gas, and more than half of it comes from humans. The most common sources are cattle and the production of fossil fuels.
Methane has been successfully removed from the air using a novel approach developed by a team at the Department of Chemistry at the University of Copenhagen and the spin-off company Ambient Carbon.
“A huge piece of our methane outflows comes from a large number of low-focus point sources like steers and pig outbuildings. By and by, methane from these sources has been difficult to move into more significant levels or eliminate. Yet, our new outcome demonstrates that it is conceivable to utilize the response chamber that we’ve assembled,” says Matthew Stanley Johnson, the UCPH barometrical science teacher who drove the review.
“Millions of low-concentration point sources, such as cattle and pig barns, contribute significantly to our methane emissions. In practice, it has been impossible to concentrate or eliminate methane from these sources. However, our recent study shows that it is doable utilizing the reaction chamber that we created.”
Matthew Stanley Johnson, the UCPH atmospheric chemistry professor who led the study.
Prior, Johnson introduced the examination results at COP 28 in Dubai through a web-based association and in Washington, D.C., at the Public Foundation of Sciences, which prompts the US government to focus on science and innovation.
Methane can be burned out of the air by a reactor if its concentration is greater than 4%. In any case, most human-caused emanations are below 0.1% and along these lines unfit to be singed.
The scientists fabricated a response chamber and contrived a strategy that mimics and significantly speeds up methane’s normal debasement process. Credit: Michael Skov Jensen, SCIENCE/KU
To eliminate methane from the air, the scientists fabricated a response chamber that, to the unenlightened, seems to be a prolonged metal box with loads of hoses and estimating instruments. A series of chemical reactions takes place inside the box, breaking down the methane and removing a significant amount of the gas from the air.
“In the logical review, we’ve demonstrated that our response chamber can wipe out 58% of methane from the air. What’s more, since presenting the review, we have worked on our outcomes in the research center with the goal that the response chamber is currently at 88%,” says Matthew Stanley Johnson.
Chlorine is vital to the revelation. Utilizing chlorine and the energy from light, scientists can eliminate methane from the air significantly more effectively than in the manner in which it occurs in the climate, where the cycle regularly requires 10–12 years.
“Methane decomposes slowly because the gas doesn’t like interacting with other things in the air. However, Johnson explains, “We have discovered that, with the assistance of chlorine and light, we can trigger a reaction and break down the methane approximately 100 million times faster than in nature.”
Up next: The Department of Chemistry will soon receive a 40-foot shipping container for livestock stalls, biogas plants, and wastewater treatment facilities. When it does, it will end up being a bigger model of the response chamber in which the specialists worked in the research center. It will be “methane more clean,” which, on a fundamental level, will actually want to be associated with the ventilation framework in a domesticated animal horse shelter.
“Landscaping farms today are high-tech facilities that already remove ammonia from the air. Therefore, the obvious solution is to remove methane through existing air purification systems,” Professor Johnson explains.

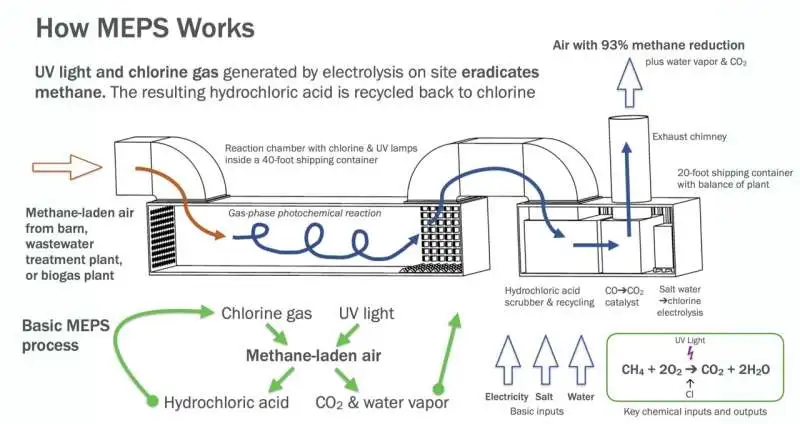
How MEPS operate. UV light and chlorine gas produced by electrolysis kill methane on location. Credit: Matthew Stanley Johnson, Division of Science, College of Copenhagen.
A similar applies to biogas and wastewater treatment plants, which are probably the biggest human-made wellsprings of methane emanations in Denmark after their creation.
The researchers measured the amount of methane that leaks from cattle stalls, wastewater treatment plants, and biogas plants as part of their preliminary investigation for this study. In a few places, the specialists had the option to report that a lot of methane spills into the environment from these plants.
“Denmark, for instance, is a pioneer in the production of biogas. Be that as it may, on the off chance that only a couple of percent of the methane from this cycle gets away, it checks any environmental gains,” finishes up Johnson.
The examination was directed in a joint effort between the College of Copenhagen, Aarhus College, Arla, Skov, and the UCPH turnout organization Surrounding Carbon, presently headed by Teacher Matthew Stanley Johnson. The organization was begun to foster MEPS (Methane Annihilation Photochemical Framework) innovation and make it accessible to society.
Concerning the method, the researchers devised a method that imitates and greatly accelerates the natural degradation of methane and constructed a reaction chamber.
MEPS reactor. Credit: Matthew Stanley Johnson, Division of Science, College of Copenhagen.
They named the technique the Methane Destruction Photochemical Framework (MEPS), and it debases methane 100 million times quicker than in nature.
The technique works by bringing chlorine particles into a response chamber with methane gas. The chlorine molecules are then illuminated by UV light from the researchers. The light’s energy makes the particles split and structure two chlorine molecules.
The chlorine particles then, at that point, take a hydrogen iota from the methane, which then self-destructs and disintegrates. The chlorine item (hydrochloric corrosive) is caught and, in this manner, reused in the chamber.
Similar to the natural process that takes place in the atmosphere, the methane transforms into carbon dioxide (CO2), carbon monoxide (CO), and hydrogen (H2) during the process.
More about methane (CH4)
Methane can be sent off to eliminate it from the air; however, its fixation should be more than 4%, or 40,000 sections, for each million ppm to be combustible. The majority of emissions caused by humans cannot be burned because they are below 1%.
The Intergovernmental Board on Environmental Change (IPCC) has confirmed that lessening methane gas emanations will promptly decrease the climb in worldwide temperatures.
Methane is a greenhouse gas that comes from natural sources like wetlands and man-made ones like food production, natural gas production, and sewage treatment facilities.
Today, methane gas is responsible for 33% of the ozone-depleting substances that influence the environment and cause a worldwide temperature alteration.
Methane decomposes naturally in the atmosphere over a period of 10 to 12 years before becoming carbon dioxide.
Methane is 85 times worse for the climate than CO2 over a 25-year period. Methane is 30 times worse for the climate than CO2 over a 100-year period.
Since the middle of the 1700s, the atmospheric concentration of methane has increased by 150 percent.
According to the IPCC, methane alone has increased anthropogenic radiation exposure by 1.19 W/m2, resulting in a 0.6°C rise in global average surface air temperature.
More information: Morten Krogsbøll et al. A high-efficiency gas-phase photoreactor for the eradication of methane from low-concentration sources, Environmental Research Letters (2023). DOI: 10.1088/1748-9326/ad0e33





