Despite the fact that there has been huge advancement in the therapy of uncommon types of blood disease lately and new medications have been endorsed in Germany, the outlook for the majority of impacted people remains ominous. Research groups at Leipzig University’s Faculty of Medicine are dealing with a few preclinical and translational tasks to acquire a superior understanding of these illnesses. The analysts are exploring how bone marrow disease creates Moreover, they have tracked down another sub-atomic useful system and have shown that patients with even low measures of leukemia cells have a high gamble of backsliding.
For what reason do up to 80% of patients with bone marrow disease (various myeloma) foster bone harm (osteolysis) in certain locales of the body while others escape this harm? This is an inquiry that has not yet been adequately examined. On an exploration stay in Buffalo, New York, the senior doctor PD Dr. Maximilian Merz from Leipzig had the option to show that the harmful plasma cells that trigger bone harm vary altogether from the other cells in the body. Myeloma cells were isolated from osteolyses and concentrated on using single-cell sequencing and other techniques.The examples obtained were then contrasted with harmful plasma cells from the iliac peak. A bone marrow biopsy is normally performed here in routine diagnostics to additionally explore the harmful cells. Dr. Merz and his group had the option of showing that plasma cells from osteolyses evoke qualities that, in addition to other things, animate bone-debasing osteoclasts and prompt new vessel arrangement. “Our discoveries highlight the way that myeloma can have many appearances and that a few changed clones can happen all the time in various areas of patients.” “This will have analytic, prognostic, and helpful results from now on,” said Dr. Merz, summing up the discoveries.
“Our findings help us better understand acute leukemia and can be utilized to optimize treatment planning. They provide a crucial foundation for the development of appropriate treatments capable of entirely eradicating even minute numbers of remaining tumor cells,”
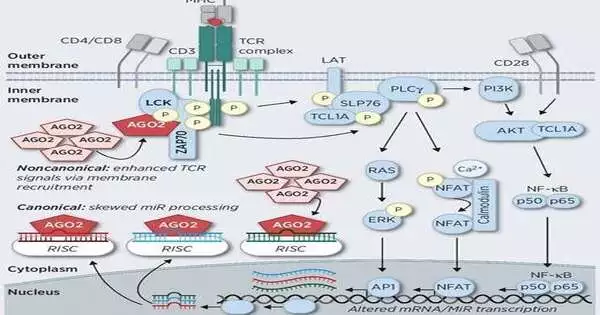
A new useful system in leukemia of T-lymphocytes was examined.
The exploration group was driven by PD. Dr. Marco Herling discovered a new, subatomic useful system for a rare and previously unknown type of T-lymphocyte blood disease.This illness seldom responds to the typical blood disease treatments and has an unfortunate guess with a typical patient endurance pace of around two years. In their examination, Herling’s group found that the particle AGO2 plays a vital part in this uncommon type of blood disease. AGO2 was recently remembered to be a key disease starting particle that was essentially engaged with the fine guideline of typical and growth quality articulation.
“In our exploration, we had the option to unravel a new, flighty useful system of AGO2 in the leukemia cells,” Dr. Herling said and afterward added, “AGO2 connects straightforwardly with fundamental proteins of signal transduction from a focal development element of the T-cell, the T-cell antigen receptor.” “The presence of AGO2 expands the reaction to approaching cell flags.” This already obscure atomic connection was approved utilizing different strategies for protein examination and its high sub-atomicity is not set in stone. This addresses another clinical way to deal with helpful mediation.
Patients with even trace amounts of leukemia cells face a high risk of relapse.
For patients with intense myeloid leukemia, bone marrow transplantation is a viable therapy choice in specific circumstances. Doctors must recognize patients who have a high chance of backsliding after transplantation as soon as conceivable during the treatment cycle. With this information, they can then change the treatment choices in the beginning phase.
The exploration group, led by PD Dr. Madlen Jentzsch and PD Dr. Sebastian Schwind, is dealing with ways of identifying even the littlest measures of enduring cancer cells in the blood and bone marrow during and after therapy for leukemia. In their review, the scientists had the option to show that the location of quantifiable leftover illness in clearly sans leukemia patients who are going to undergo bone marrow transplantation is a significant prognostic element. Leftover illness is pertinent whether or not the transplantation was carried out as an underlying treatment or solely after the patient had backslid. “Our outcomes assist us with bettering our grasp of intense leukemia and can be utilized to improve therapy arrangements,” said PD Dr. Jentzsch. “They give a significant premise to the improvement of proper medicines that are able to totally kill even momentary measures of enduring cancer cells,” she added.
More information: Till Braun et al, Noncanonical Function of AGO2 Augments T-cell Receptor Signaling in T-cell Prolymphocytic Leukemia, Cancer Research (2022). DOI: 10.1158/0008-5472.can-21-1908
Madlen Jentzsch et al, Impact of the MRD status in AML patients undergoing allogeneic stem cell transplantation in first vs second remission, Blood Advances (2022). DOI: 10.1182/bloodadvances.2022007168