Specialists at the University of Toronto, Johns Hopkins University, and Vanderbilt University have found that specific cells move shockingly quicker in thicker liquids—think honey rather than water, or bodily fluid instead of blood—in light of the fact that their unsettled edges sense the consistency of their current circumstance and adjust to speed up.
Their joined outcomes in malignant growth and fibroblast cells—the sort that frequently makes scars in tissues—propose that the thickness of a phone’s general climate is a significant supporter of sickness, and may assist with making sense of cancer movement, scarring in bodily fluid-filled lungs impacted by cystic fibrosis, and the injury recuperating process.
The review, “Layer unsettling is a mechanosensor of extracellular liquid consistency,” distributed today in Nature Physics, reveals new insight into cell conditions, an under-investigated area of examination.
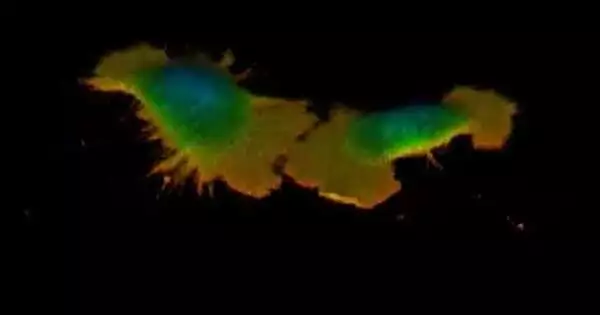
3D render of an exceptionally metastasized, “unsettled” bosom malignant growth cell (MDA-MB-231 cell line), spreading upon the expansion of gooey medium. Gooey medium was added at 10:55. Variety is coded for level, where cooler tones are higher. The video is displayed at 25 frames per second.
“This connection between cell thickness and connection has never been illustrated,” says Sergey Plotnikov, right-hand teacher in the Department of Cell and Systems Biology in the Faculty of Arts and Science at the University of Toronto and a co-creator of the review. “We found that the thicker the general climate, the more grounded the cells stick to the substrate and the quicker they move—similar to strolling on a cold surface with shoes that have spikes, versus shoes with no hold by any means.”
“By showing how cells respond to what’s around them, and by describing the physical properties of this area, we can learn what affects their behavior and eventually how to influence it,”
Ernest Iu, Ph.D. student in the Department of Cell and Systems Biology
Understanding the reason why cells act in this astounding manner is significant in light of the fact that disease growths establish a gooey climate, and that implies spreading cells can move into growths quicker than non-carcinogenic tissues. Since the scientists saw that malignant growth cells accelerate in a thickened environment, they reasoned that the improvement of unsettled edges in disease cells might contribute to malignant growth spreading to different regions of the body.
Human Embryonic Kidney cells (HEK-293 cell line) spreading in thick medium in 3D.Thick medium was added at 16:30. Variety is coded for level, where cooler tones are higher. The video is displayed at 25 frames per second.
Focusing on the spread reaction in fibroblasts, then again, may lessen tissue harm in the bodily fluid-filled lungs impacted by cystic fibrosis. Since unsettled fibroblasts move rapidly, they are the main sort of cell to travel through the bodily fluid to the injury, adding to scarring instead of recuperating. These outcomes additionally suggest that by changing the consistency of the lung’s bodily fluid, one has some control over the cell development.
By showing how cells answer what’s around them and by portraying the actual properties of this area, we can realize what influences their way of behaving and, in the end, how to impact it,” says Ernest Iu, Ph.D. understudy in the Department of Cell and Systems Biology in the Faculty of Arts and Science at the University of Toronto and study co-creator.
Plotnikov adds, “For instance, maybe in the event that you put a fluid as thick as honey into an injury, the cells will move further and quicker into it, consequently mending it all the more.”
Plotnikov and Iu utilized advanced microscopy procedures to quantify the foothold that cells apply to moving and changes in primary atoms inside the cells. They looked at malignant growth and fibroblast cells, which have unsettled edges, and cells with smooth edges. They discovered that unsettled cell edges sense the thickened climate, setting off a reaction that permits the cell to get through the obstruction — the unsettles straighten down, spread out, and hook on to the encompassing surface.
The examination started at Johns Hopkins, where Yun Chen, associate teacher in the Department of Mechanical Engineering and lead creator of the review, and Matthew Pittman, Ph.D. understudy and first creator, were first analyzing the development of disease cells. Pittman made a gooey, bodily fluid like polymer arrangement, kept it on various cell types, and saw that malignant growth cells moved quicker than non-harmful cells while relocating through the thick fluid. To further test this way of behaving, Chen worked together with U of T’s Plotnikov, who has practical experience in the back and forth of cell development.
Plotnikov was astonished at the adjustment of speed going into thick, bodily fluid-like fluid. “Ordinarily, we’re taking a gander at slow, unobtrusive changes under the magnifying lens, but we could consider the cells moving two times to be quick and continuous, and spread to twofold their unique size,” he says.
Regularly, cell development relies upon myosin proteins, which assist muscles with contracting. Plotnikov and Iu contemplated that halting myosin would keep cells from spreading, but were amazed when proof showed the cells actually accelerated notwithstanding this activity. They instead found that segments of the actin protein inside the cell, which contribute to muscle withdrawal, turned out to be more steady in light of the thick fluid, further pushing out the edge of the cell.
The groups are currently examining how to slow the development of unsettled cells through thickened conditions, which might pave the way for new therapies for individuals impacted by malignant growth and cystic fibrosis.
More information: Jian Liu, Membrane ruffling is a mechanosensor of extracellular fluid viscosity, Nature Physics (2022). DOI: 10.1038/s41567-022-01676-y. www.nature.com/articles/s41567-022-01676-y
Journal information: Nature Physics