A threat to global health is antibiotic resistance. An estimated 1.3 million deaths worldwide in 2019 were caused by antibiotic-resistant bacterial infections. Researchers at Baylor College of Medicine have been examining the molecular mechanism that underlies antibiotic resistance in an effort to contribute to a solution to this growing issue.
They document in the journal Molecular Cell important and unexpected initial steps that contribute to resistance to ciprofloxacin, also known as cipro or cipro, one of the most frequently prescribed antibiotics. The research suggests potential methods for preventing bacterial resistance, thereby preserving the potency of both new and old antibiotics.
“Past work in our lab has shown that when bacteria are subjected to a stressful environment, such as the presence of cipro, they launch a sequence of responses in an attempt to survive the antibiotic’s damaging effect,”
Author Dr. Susan M. Rosenberg, Ben F. Love Chair in Cancer Research
The co-corresponding author, Dr. Susan M. Rosenberg, said, “Previous work in our lab has shown that when bacteria are exposed to a stressful environment, such as the presence of cipro, they initiate a series of responses in an attempt to survive the toxic effects of the antibiotic. Professor of molecular and human genetics, biochemistry and molecular biology, molecular virology, and molecular microbiology at Baylor and the Love Chair in Cancer Research. She also serves as the program director at the Dan L. Duncan Comprehensive Cancer Center (DLDCCC) at Baylor.
“We discovered that cipro causes cellular stress reactions that encourage mutations. Stress-induced mutagenesis, as this phenomenon is also known, produces mutant bacteria, some of which are cipro-resistant. Growing cipro-resistant mutants maintain an infection that is no longer treatable with cipro.”.
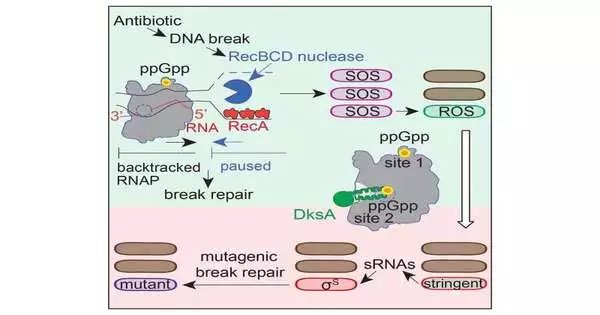
Cipro causes DNA molecule breaks, which build up inside bacteria and ultimately cause a DNA damage response to be triggered in order to repair the breaks. Two stress responses, the general stress response and the DNA-damage response, are critical in the process of stress-induced mutagenesis, as discovered by the Rosenberg lab.
The Rosenberg lab and her colleagues have previously identified some of the downstream steps of the procedure that result in increased mutagenesis. The first steps between an antibiotic breaking DNA and the bacterium activating the DNA damage response were uncovered by the researchers in this study’s molecular mechanisms.
The first author, Dr. Yin Zhai, a postdoctoral associate in the Rosenberg lab, said, “We were surprised to find an unexpected molecule involved in modulating DNA repair.”. “Typically, proteins that mediate the desired function are produced by cells to control their activities. However, in this instance, activating particular genes that result in specific proteins was not the first step in starting the DNA repair response.
Instead, the first step involved inhibiting the function of an already-present protein called RNA polymerase. To synthesize proteins, RNA polymerase is essential. In order to translate DNA-encoded instructions into an RNA sequence, which is then translated into a protein, this enzyme binds to DNA.
According to Zhai, “We found that RNA polymerase also plays a significant role in regulating DNA repair.”. “A small molecule called nucleotide ppGpp, which is present in bacteria exposed to a stressful environment, binds to RNA polymerase through two different sites that are crucial for activating the repair response and the general stress response. When one of these sites is compromised, DNA repair specifically at the DNA sequences occupied by RNA polymerase is turned off.”.
Dr. Christophe Herman, co-corresponding author and professor of molecular and human genetics, molecular virology, and microbiology, and member of the DLDCCCC, explained that ppGpp binds to DNA-bound RNA polymerase and instructs it to stop and backtrack along the DNA to repair it. The connection between repair and RNA polymerase was previously discovered by the Herman lab and published in Nature.
DNA repair can be an error-prone process, as Rosenberg’s lab found. Errors arise during the process of repairing the broken DNA strands, changing the original DNA sequence, and leading to mutations. A few of these mutations will give bacteria Cipro resistance. It’s interesting that the mutations also cause the bacteria to become resistant to two additional antibiotics, according to Zhai.
Rosenberg expressed his excitement about the results. They provide new opportunities for developing tactics that would thwart the rise of antibiotic resistance and aid in the fight against this threat to global health. Cipro also destroys bacterial DNA in a manner similar to that of etoposide, an anti-cancer medication that destroys human DNA in tumors. Additionally, we hope that this may result in new tools to combat cancer chemotherapy resistance.
More information: Yin Zhai et al, ppGpp and RNA-polymerase backtracking guide antibiotic-induced mutable gambler cells, Molecular Cell (2023). DOI: 10.1016/j.molcel.2023.03.003