Locally established nonstop glucose testing for diabetics has previously had to trade usability, low cost, and conveyability for a significantly lower responsiveness—and thus, exactness—when compared to comparative frameworks in facilities or clinics.A group of scientists has now developed a biosensor for such screens that includes “zero-layered” quantum specks (QDs) and gold nanospheres (AuNSs) and no longer needs to think twice about exactness.
A paper portraying the biosensor plan and its improved exhibition showed up in the journal Nano Exploration on November 9, 2022.
Lately, the improvement of constant glucose monitoring (CGM) technology has been an extraordinary shelter for individuals with diabetes. Unlike pre-meal and pre-sleep glucose testing, continuous, fast, and accurate recognition of glucose levels on CGM devices has completely improved diabetic management.
Glucose patterns are all the more effortlessly followed, making diet, exercise, and medication changes to a diabetes care plan simpler to execute over the course of the day, and cautions go off when glucose levels climb excessively high or fall excessively low, sending data to the individual or to guardians, accomplices, or parental figures.
“It also has adequate but not exceptional sensitivity. Not when compared to other approaches utilized in a medical setting. So we wanted to test if we could increase the sensitivity and so enhance the accuracy.”
Huan Liu, a microelectronics specialist with the School of Optical and Electronic Information
CGMs commonly work through a little biosensor implanted under the skin that measures glucose levels in the liquid between cells. This sensor really takes a look at such levels at regular intervals and sends that data to a screen. The screen can likewise be associated with an insulin siphon.
Different strategies for glucose discovery have been created, including colorimetry, infrared spectroscopy, fluorescence spectroscopy, and mass spectrometry. However, for locally established activities instead of at a facility or medical clinic, electrochemical glucose identification is the most generally acknowledged procedure because of its fast reaction, convenience, minimal expense, and movability.
“It likewise has fair responsiveness, yet not magnificent awareness,” said Huan Liu, a microelectronics expert with the School of Optical and Electronic Data at Huazhong College of Science and Innovation. “Not in comparison to other strategies used in a medical care setting.”So we needed to see if we could give that responsiveness a little boost and then work on its exactness.
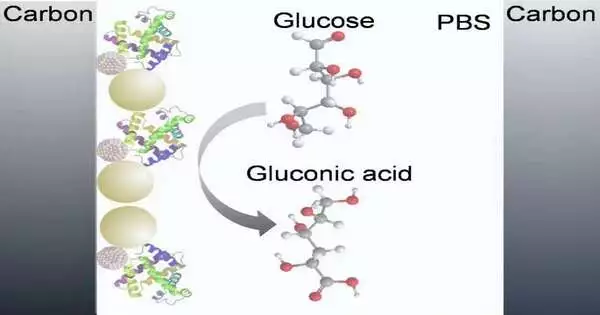
Electrochemical glucose sensors can be categorized as catalyst-based sensors or non-protein-based sensors. For the catalyst-based glucose electrochemical sensors, glucose oxidase (GOx)—a compound that velocises up (catalyzes) oxidation-decrease substance responses—is broadly used to oxidize glucose on the outer layer of the CGM sensor terminal.
The terminal draws electrons from the glucose (oxidizing them) and in the process produces an electric flow that shifts depending upon glucose levels. GOx is generally utilized for this reason because of its high selectivity for glucose (the capacity to choose for glucose and not different substances), high steadiness, and high action over an extensive variety of pH levels.
In any case, when GOx is straightforwardly joined with the uncovered terminal surface, not just GOx itself is effortlessly shed (deprived of a portion of its layers), yet its natural movement and security can likewise be impacted. What’s more, electron-movement productivity between the GOx and the cathode surface is a key variable deciding the responsiveness of the sensor.
Various attempts have been made up to this point to make the GOx compound more solidly attached to the terminal, thereby improving the immediate electron move between the electroactive focuses (locations of electron action) and the anode surface.One outstanding endeavor includes the utilization of cathodes planned at the nanoscale to have structures on the terminal that give bigger surface regions and high electrocatalytic action.
Sadly, these nanostructures increase the complexity of creating such electrochemical biosensors. Their development likewise depends on the engineered polymer Nafion as a framework, which makes an obstruction for the charge to move across the connection point between the sensor and the liquid being tested.
The scientists have thusly headed somewhere else altogether. The group pointed toward further developing glucose detection by utilizing colloidal quantum dots (CQDs) as the material for changing the cathode. CQDs are “zero”-layered semiconductor nanoparticles. (They are not really zero aspects, but instead incredibly little measurements regularly going from 2 to 20 nm.) These have a plethora of dynamic destinations—where compound responses can occur—and are inextricably linked to natural protein particles.
Far and away superior, because of their exceptionally small size, CQDs go through quantum impacts (for example, quantum burrowing), and the charge movement at the CQD-protein point of interaction can be controlled by the use of an outer electric field. CQDs can also be made with a variety of rigid and adaptable substrate materials, making them more efficiently manufactured.
Improving this impact, the specialists incorporated gold nanospheres (AuNSs) into the design of the sensor cathode. These are very small circular nanoparticles, with measurements ranging from 10–200 nm. They are progressively being utilized in biosensing applications because of their exceptional physical and synthetic properties.
Specifically, when utilized as a part in enzymatic electrochemical biosensors, AuNSs permit protein chemicals to hold their natural movement upon gripping surfaces and diminish the protective impact of the protein shell for direct electron movement. In a CGM, this enormously improves the signal sufficiency of the electrochemical biosensors.
The specialists developed a proof-of-concept CGM utilizing CQDs—ffor this situation, made of lead sulfide—aand the AuNSs-changed terminal. They discovered that the expansion of the AuNSs, as expected, worked fundamentally on the ongoing sign identified by the electrochemical sensor.
Consolidated, these changes showed extraordinary potential in distinguishing glucose in various examples like blood, sweat, and other natural liquids and conveyed a quick (under 30 seconds) electrochemical biosensor with a wide discovery range and the kind of super-high responsiveness the group was looking for.
The scientists currently expect to take their verification idea for CGM and make it manufacturable at business scale.
More information: Yunong Zhao et al, Electrochemical biosensor employing PbS colloidal quantum dots/Au nanospheres-modified electrode for ultrasensitive glucose detection, Nano Research (2022). DOI: 10.1007/s12274-022-5138-0
Journal information: Nano Research